A new drug that attacks the precise form of deadly brain cancer that has stricken GOP Sen. John McCain of Arizona is bein...
 Oops! We couldn’t find any results...
Oops! We couldn’t find any results...
 Oops! We couldn’t find any results...
Oops! We couldn’t find any results...
CNS Pharma

Open for investment
Documents
 Form C
SEC.gov
Form C
SEC.gov
Deal highlights
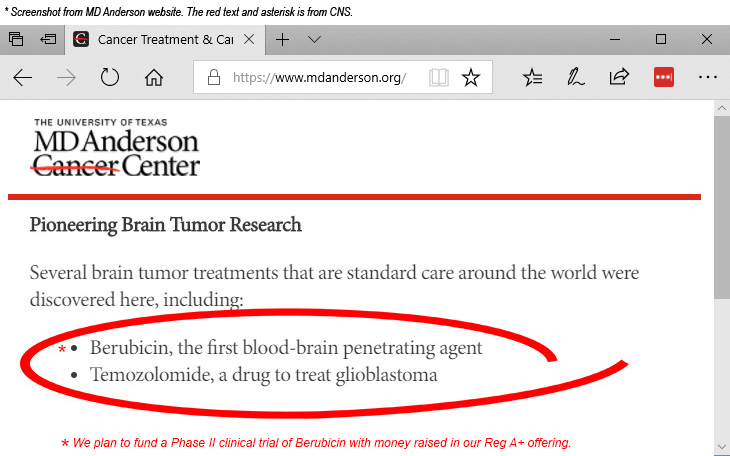
- CNS is developing Berubicin, a breakthrough drug for the treatment of the most deadly form of brain cancer.
- Berubicin was created at MD Anderson Cancer Center, the largest cancer and research institute in the world.
- Berubicin’s Phase I clinical trial yielded promising results showing clinical activity in 44% of patients*.
- Berubicin is the first of its class of drugs to cross the blood brain barrier (BBB) and reach cancer cells in brain tumor patients.
- Led by industry leading scientists and executives with decades of experience developing drugs and bringing medical products to market.
- Signed a collaboration agreement with Reata Pharmaceuticals, a $700M+ NASDAQ listed company, to further advance the development of brain cancer technologies.
- CNS intends to list on the NASDAQ in 2018.
*Study not designed to demonstrate safety and effectiveness
78,980 Reasons Why Our Focus Is The Brain
In the United States, an estimated 78,980 new cases of brain tumor are expected to be diagnosed in 2018. Of these, the deadliest form is Glioblastoma Multiforme (GBM), responsible for the highest number of cases of all malignant tumors, with 12,500 cases projected in 2017 and 12,760 in 2018.*
Glioblastoma is the type of brain cancer that killed Senator Ted Kennedy and Vice President Joe Biden’s son. Unfortunately, Senator John McCain was also recently diagnosed with this deadly disease.

GBM is the most aggressive and common primary brain cancer in adults. It is highly invasive, virtually incurable, and the primary target for our lead drug candidate Berubicin.
Despite decades of research, the survival outcomes for patients with GBM remain virtually unchanged, with a median survival time of 14.6 months. * CBTRUS report
Berubicin | A New Approach To Treatment

Berubicin, was created at the MD Anderson Cancer Center by Dr. Waldemar Priebe. Dr. Priebe is the founder of CNS Pharmaceuticals.
Berubicin is considered a breakthrough technology because...

What is an anthracycline?
An anthracycline is a class of drugs that are among the most effective anticancer treatments ever developed and are effective against more types of cancer than any other class of chemotherapeutic agents for cancers like breast, ovarian, leukemia, lymphoma, testicular, and others.
While this class of drugs has been extremely successful at treating even the most aggressive types of cancers, unfortunately in over 60 years of clinical research, anthracyclines have NEVER been shown to cross the blood-brain barrier (BBB) and impact deadly brain cancers… until now.
The very promising results of the Phase I clinical trial of Berubicin in GBM patients, completed by Reata Pharmaceuticals, demonstrated significant anti-tumor activity in 44% of the treated patients.*
It consisted of 61 patients which were enrolled in two separate studies. Both dose finding and safety were studied in patients with GBM or other brain cancers.
Berubicin has also been granted Orphan Drug status by the FDA in the US.
In 2018, with the funding to be secured from this equity crowdfunding campaign and a planned NASDAQ IPO, CNS expects to commence its Phase 2(a) clinical trial of Berubicin for the treatment of GBM.
In late 2017, CNS entered into a collaboration agreement with Reata Pharmaceuticals, currently a $700M+ NASDAQ listed company, to further advance the development of brain cancer technologies.

*Based on all tumor cell lines so far tested, Berubicin has been significantly more potent than doxorubicin, more cytotoxic, and a more potent topoisomerase II poison.
Example of Berubicin Phase I Clinical Trial Results
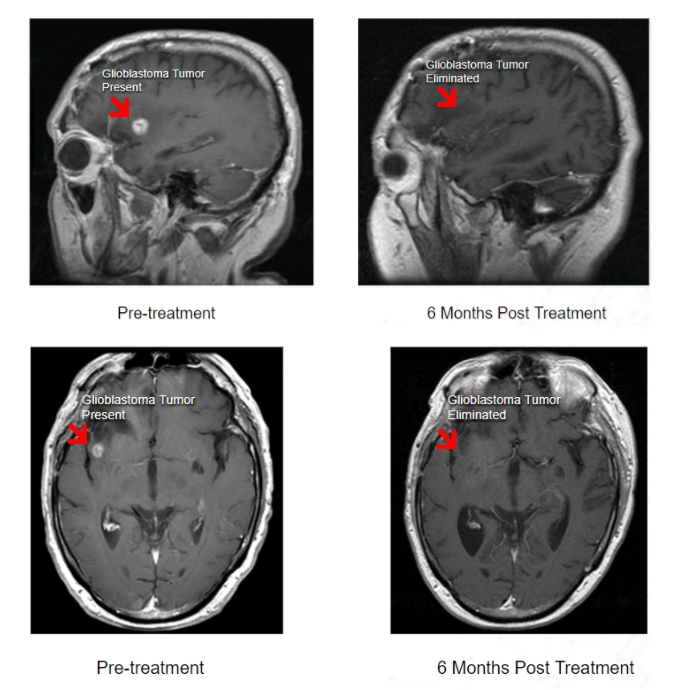
*Study not designed to demonstrate safety and effectiveness
Watch Berubicin Cross The Blood Brain Barrier
At 2 minutes and 44 seconds into the video above, there is an animation that will show you how Berubicin is able to cross the BBB and attack a tumor.
What's Currently Being Used To Fight GBM?
The current standard for treatment for GBM is surgery, radiation, and chemotherapy with Temozolomide (TMZ). TMZ, the current standard of treatment for GBM, has limited efficacy.

Drugs currently used for the treatment of other cancers are not effective for the treatment of brain tumors. The brain’s protective mechanism, the BBB, is responsible for shielding brain tumors from the existing and potential new anti-cancer agents. The BBB makes development of effective drugs for brain tumors very difficult.
The lack of progress in the treatment of GBM provides a tremendous opportunity to identify better drugs like Berubicin.
Market Opportunity
If approved, Berubicin has the potential to realize a multi-billion dollar opportunity as a stand-alone or combination therapy for GBM and other cancers.
Approximately 40% of GBM patients have a genetic variation, which makes their tumors initially more responsive to Temozolomide (TMZ). TMZ is the current standard of care for these patients. Nearly all of these patients will quickly become resistant to TMZ. Berubicin could be prescribed after TMZ’s failure.
In case of the remaining 60% of GBM patients, TMZ is ineffective and Berubicin could be prescribed as a primary drug treatment.

In 2009, Schering reported worldwide sales of temozolomide (TMZ) of $1 billion. Current numbers for temozolomide market share may be lower since launch of generics in 2013.
As of now, there is no standard of care for recurrent GBM. Bevacizumab (Avastin) is a recently approved by FDA drug for chemotherapy for glioblastoma at relapse; however it does not improve survival.
Short-term efficacy of the current standard of treatment and low survival rate of GBM patients and other related central nervous system malignancies, create a significant unmet need and financial opportunity.
Berubicin has the potential to become the standard of care treatment for recurrent and TMZ resistant GBM. CNS will plan future clinical trials to establish Berubicin as an upfront treatment for glioblastoma. If approved, CNS believes that Berubicin has the potential to realize a multi-billion dollar opportunity as a stand-alone or combination therapy for GBM and other cancers.
Development Pipeline

Management

John M. Climaco, JD is the CEO of CNS Pharmaceuticals, Inc. For 15 years Mr. Climaco has served in leadership roles in a variety of healthcare companies. Recently Mr. Climaco served as the Executive Vice-President of Perma-Fix Medical S.A where he managed the development of a novel method to produce Technitium-99. Previously Mr. Climaco served as President and CEO of Axial Biotech, Inc., a DNA diagnostics company. In the process of taking Axial from inception to product development to commercialization, Mr. Climaco created strategic partnerships with Medtronic, Johnson & Johnson and Smith & Nephew. Mr. Climaco currently serves as a director of several public companies including Moleculin Biotech, Inc., pharmaceutical company focused on anti-cancer drug candidates. Mr. Climaco also served as a director of PDI, Inc., a provider of outsourced commercial services to pharma companies, and InfuSystem Holdings, Inc., the largest supplier of infusion services to oncologists in the US.
Sandra L. Silberman, M.D., Ph.D. is the Chief Medical Officer of CNS Pharmaceuticals. Dr. Silberman is a Hematologist/Oncologist who earned her B.A., Sc.M. and Ph.D. from the Johns Hopkins University School of Arts and Sciences, School of Public Health and School of Medicine, respectively, and her M.D. from Cornell University Medical College, and then completed both a clinical fellowship in Hematology/Oncology as well as a research fellowship in tumor immunology at the Brigham & Women's Hospital and the Dana Farber Cancer Institute in Boston, MA. Dr. Silberman has played key roles in the development of many drugs including Gleevec™, for which she led the global clinical development at Novartis. Dr. Silberman advanced several original, proprietary compounds into Phases I through III during her work with leading biopharmaceutical companies, including Bristol-Myers Squibb, AstraZeneca, Imclone and Roche.
Matt Lourie, CPA is the CFO of CNS Pharmaceuticals, Inc. Mr. Lourie has extensive management, accounting and financial experience. Mr. Lourie served as an audit partner of the PCAOB registered firm MaloneBailey where he oversaw audits and financial reporting of SEC registrants. In addition, he served as the Corporate Controller of a public company with over 300 locations across the country. Mr. Lourie is a graduate of the University of Houston where he earned both his Bachelor of Business Administration - Accounting and his Masters of Science in Accounting.
Board & Scientific Advisory Board

Donald Picker, PhD, joined the CNS team in November, 2017 with over 35 years of drug development experience. At Johnson Matthey, Dr. Picker was responsible for the development of Carboplatin, one of the world’s leading cancer drugs, acquired by Bristol-Myers Squibb and with annual sales of over $500 million. He also oversaw the development of Satraplatin and Picoplatin, third-generation platinum drugs currently in late-stage clinical development. Dr. Picker has significant experience in dermatological pharmaceutical discovery and development as well, having led projects for topical therapies in psoriasis, atopic dermatitis and acne.
J. George Gumulka, PhD, has more than 30 years of industrial and academic experience primarily in the R&D functions. George is an accomplished technology leader with strong international experience and with an excellent track record of successful new product and application. He has led innovation, technology and supported business efforts at several major global chemical companies including., Royal Dutch Shell/Shell Chemical Company, Kraton Polymers U.S. LLC, and Biospectrum Inc. His experience crosses multiple global industrial sectors including biotechnology, polymer and elastomer applications in consumer products, electronics, general industrial, commercial and residential construction, oil transportation, water purification to name a few.
Waldemar Priebe, PhD, Chairman of the Scientific Advisory Board, is a world renowned medicinal chemist and entrepreneur. Dr. Priebe is a Professor of Medicinal Chemistry in the Section of Immunobiology and Drug Carriers in the Department of Bioimmunotherapy at MD Anderson. Dr. Priebe is the inventor of more than 50 patents and the author of more than 200 scientific publications. As the founder or founding scientist of 6 pharmaceutical companies, including three listed on NASDAQ, Dr. Priebe has been integral in advancing several drugs through the pipeline, five of which entered clinical development. Dr. Priebe led the research that formed basis for the development of agents with high brain uptake (BBB crossing) and is the discoverer of our lead drug candidate Berubicin.
Sigmund Hsu, MD is fellowship trained and certified by the American Board of Psychiatry and Neurology, with extensive experience in the evaluation and treatment of neurological disorders in cancer patients. He specializes in primary brain tumors as well as brain and spinal cord metastases, cancer neurology and the treatment of chemotherapy neurotoxicity. Dr. Hsu has presented research at several national conferences, and his work has been published in numerous journals and textbooks. His most recent research has focused on novel therapies for recurrent primary CNS lymphoma, recurrent glioblastoma multiforme and intralumbar injections for cancer therapy, and he has several patents granted and pending for his treatments.
Possible IPO / Exit

Our first step is to raise the maximum funding allowed on Republic, which is $1,070,000. This is the campaign that you are currently viewing now.
Shortly after, we plan to begin a Regulation A+ equity crowdfunding campaign on Sprout Equity and raise up to $15,000,000.
After we close out the Reg A+ fundraising round, we plan on listing on the NASDAQ stock exchange in 2018.
Deal terms
16%
If a trigger event for CNS Pharma occurs, the discount provision
gives investors equity shares (or equal value in cash) at a reduced price.
Learn more.
$1.07M
CNS Pharma must achieve its minimum goal of $100K before the deadline. The maximum amount the offering can raise is $1.07M.
Learn more
Learn more
Crowd SAFE
A SAFE allows an investor to make a cash investment in a company, with rights to receive certain company stock at a later date, in connection with a specific event.
·
Learn more
Documents
 Form C
SEC.gov
Form C
SEC.gov
Why others invested
See all reviews (0) See all (0)My father was diagnosed with a glioblastoma and fighting! We need to find a cure and fast to beat this deadly disease. I believe this is the right steps in finding a cure!
I invested because my father died of brain cancer in 1967. My prayer ever since then has been for someone to find a cure.
I read their Form C, read up on backgrounds of founders, AND because CNS was rec by an advisory service to which I subscribe.
About CNS Pharma
CNS Pharma Team
Everyone helping build CNS Pharma, not limited to employees
Press
This site (the "Site") is owned and maintained by OpenDeal Inc., which is not a registered broker-dealer. OpenDeal Inc. does not give investment advice, endorsement, analysis or recommendations with respect to any securities. All securities listed here are being offered by, and all information included on this Site is the responsibility of, the applicable issuer of such securities. The intermediary facilitating the offering will be identified in such offering’s documentation.
All funding-portal activities are conducted by OpenDeal Portal LLC doing business as Republic, a funding portal which is registered with the US Securities and Exchange Commission (SEC) as a funding portal (Portal) and is a member of the Financial Industry Regulatory Authority (FINRA). OpenDeal Portal LLC is located at 149 E 23rd St #1314, New York, NY 10010, please check out background on FINRA’s Funding Portal page.
All broker-dealer related securities activity is conducted by OpenDeal Broker LLC, an affiliate of OpenDeal Inc. and OpenDeal Portal LLC, and a registered broker-dealer, and member of FINRA | SiPC, located at 1345 Avenue of the Americas, 15th Floor, New York, NY 10105, please check our background on FINRA’s BrokerCheck.
Certain pages discussing the mechanics and providing educational materials regarding regulation crowdfunding offerings may refer to OpenDeal Broker LLC and OpenDeal Portal LLC collectively as “Republic”, solely for explanatory purposes.
Neither OpenDeal Inc., OpenDeal Portal LLC nor OpenDeal Broker LLC make investment recommendations and no communication, through this Site or in any other medium should be construed as a recommendation for any security offered on or off this investment platform. Investment opportunities posted on this Site are private placements of securities that are not publicly traded, involve a high degree of risk, may lose value, are subject to holding period requirements and are intended for investors who do not need a liquid investment. Past performance is not indicative of future results. Investors must be able to afford the loss of their entire investment. Only qualified investors, which may be restricted to only Accredited Investors or non-U.S. persons, may invest in offerings hosted by OpenDeal Broker.
Neither OpenDeal Inc., OpenDeal Portal LLC nor OpenDeal Broker LLC, nor any of their officers, directors, agents and employees makes any warranty, express or implied, of any kind whatsoever related to the adequacy, accuracy or completeness of any information on this Site or the use of information on this site. Offers to sell securities can only be made through official offering documents that contain important information about the investment and the issuers, including risks. Investors should carefully read the offering documents. Investors should conduct their own due diligence and are encouraged to consult with their tax, legal and financial advisors.
By accessing the Site and any pages thereof, you agree to be bound by the Terms of Use and Privacy Policy. Please also see OpenDeal Broker’s Business Continuity Plan and Additional Risk Disclosures. All issuers offering securities under regulation crowdfunding as hosted by OpenDeal Portal LLC are listed on the All Companies Page. The inclusion or exclusion of an issuer on the Platform Page and/or Republic’s Homepage, which includes offerings conducted under regulation crowdfunding as well as other exemptions from registration, is not based upon any endorsement or recommendation by OpenDeal Inc, OpenDeal Portal LLC, or OpenDeal Broker LLC, nor any of their affiliates, officers, directors, agents, and employees. Rather, issuers of securities may, in their sole discretion, opt-out of being listed on the Platform Page and Homepage.
Investors should verify any issuer information they consider important before making an investment.
Investments in private companies are particularly risky and may result in total loss of invested capital. Past performance of a security or a company does not guarantee future results or returns. Only investors who understand the risks of early stage investment and who meet the Republic's investment criteria may invest.
Neither OpenDeal Inc., OpenDeal Portal LLC nor OpenDeal Broker LLC verify information provided by companies on this Site and makes no assurance as to the completeness or accuracy of any such information. Additional information about companies fundraising on the Site can be found by searching the EDGAR database, or the offering documentation located on the Site when the offering does not require an EDGAR filing.
To help the government fight the funding of terrorism and money laundering activities, Federal law requires all financial institutions to obtain, verify, and record information that identifies each person who opens an account. Therefore, when you use the Services we will ask for your name, address, date of birth, and other information that will allow us to identify you. We may also ask to see your driver's license, passport or other identifying documents.
Republic and its affiliates are not and do not operate or act as a bank. Certain banking services are provided by BankProv, member FDIC / member DIF. Digital (crypto) assets and investment products are not insured by the FDIC, may lose value, and are not deposits or other obligations of BankProv and are not guaranteed by BankProv. Terms and conditions apply.

Made in SF/NYC