15+ min read Opinions expressed by Entrepreneur contributors are their own. While the Covid-19 pandemic wreaked havoc in ...
Problem
Major depressive disorder affects more than 17M Americans
Depression is the second psychiatric disease worldwide, affecting more than 264 million people of all ages, including 17.3 million Americans, and it is a leading cause of disability in the world.
Furthermore, each year more veterans die by suicide (7,300) than the total number of US military deaths in Afghanistan and Iraq.
Covid-19 has created a new time bomb: the mental health pandemic.
Since the start of the pandemic, about 4 in 10 adults in the United States have reported symptoms of depressive disorder and anxiety, compared to one in 10 adults in 2019.
Additionally, it is now clinically proven that one in three COVID19 survivors received a neurological or psychiatric diagnosis within six months of infection, according to an observational study of more than 230,000 patient health records published in The Lancet.
Society does not have enough medical staff to treat these many patients, with today's standard of care that is time-consuming and labor-intensive and can only be performed in a hospital setting.
There simply aren't enough hospitals and clinics.
Treating depression from home offers the only solution for treating these many patients to reduce the risk of suicide and the financial burden of major depressive disorder in society.
Solution
Neuromodulation: the future of depression treatment
Non-invasive brain stimulation (or neuromodulation) has been used increasingly for decades to provide a painless and non-pharmacological (or adjuvant to) treatment to patients who are either resistant to psychiatric medication or suffer moderate to severe side effects from it.
Actipulse’s proprietary neuromodulation technology is based on the same principles of Transcranial Magnetic Stimulation (TMS), which was first approved by the FDA in 2008 for the treatment of Major Depressive Disorder.
Our patent-pending technology is an innovation on the traditional TMS, by using high-frequency and low-intensity magnetic pulses to achieve a therapeutic effect.
This has allowed us to engineer and manufacture a hospital-setting device that is smaller, earlier to use, and allows for any medical personnel to apply the therapy, not just skilled clinicians that previously needed 52 hour courses on how to apply the treatment.
Actipulse’s clinical results have been demonstrated through multiple peer-reviewed publications:
"The effectiveness of exogenous melatonin versus transcranial magnetic stimulation on the quality of sleep, memory, and mood of young adult people"
In this controlled study, patients using our proprietary neuromodulation device showed a greater reduction of anxiety symptoms from baseline than patients in the melatonin treatment, sham, or control groups; they also showed a significant improvement in sleep quality and memory span baseline.
"High-Frequency and Low-Intensity Patterned Transcranial Magnetic Stimulation over Left Dorsolateral Prefrontal Cortex as Treatment for Major Depressive Disorder"
Patients with a previous diagnosis of major depressive disorder received 15 sessions of treatment using Actipulse's proprietary neuromodulation device, after the treatment ended, all of the subjects showed significant changes in their depression, anxiety, and cognitive evaluations.
“Effect of Fast Gamma Magnetic Stimulation over the left Prefrontal Dorsolateral Cortex for the Treatment of MCI and Mild Dementia: A randomized Proof of Concept Sham-Controlled Clinical Trial.” (In review)
In this pilot trial, fast gamma neuromodulation provided by Actipulse demonstrated to be a technique that can be used safely without the supervision of a healthcare provider by this population.
“High Frequency and Low Intensity Transcranial Magnetic Stimulation for Smoking Cessation” (In review)
Neuromodulation provided by an Actipulse device elicited a decrease in depression and anxiety symptoms in most of the participants in this study; also, all subjects showed a statistically significant reduction in exhaled carbon monoxide concentrations from baseline, measures that indicate that patients had reached remission from smoking after finalizing the treatment.
Product
Bringing neuromodulation treatment for depression, from the hospital to the home
With the data from our clinical trials, market-feedback from more than 210 physicians using our hospital-setting device, and four years of experience in neuromodulation R&D, we are ready to take on the challenge of treating Major Depressive Disorder, from the comfort of patients’ homes, in the United-States.
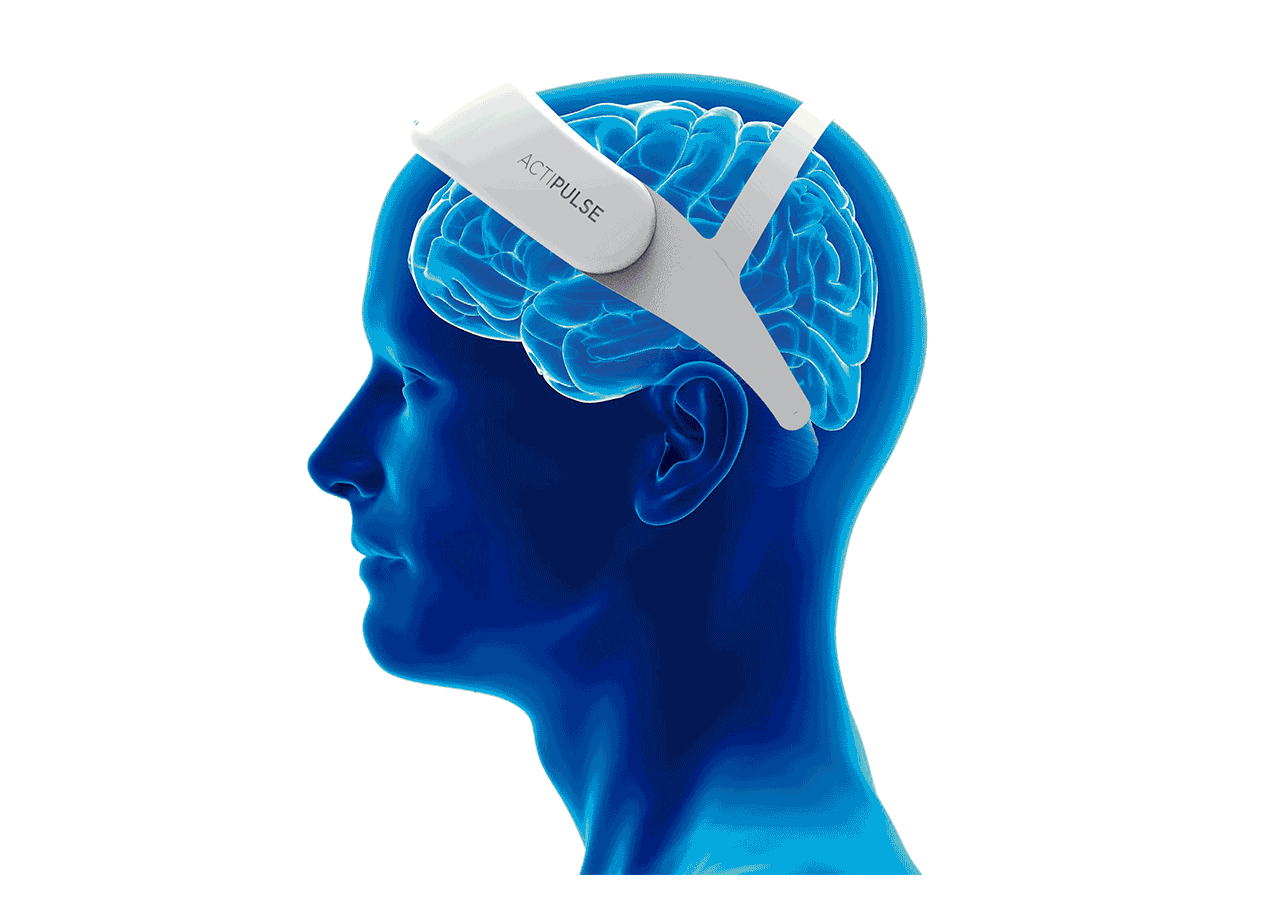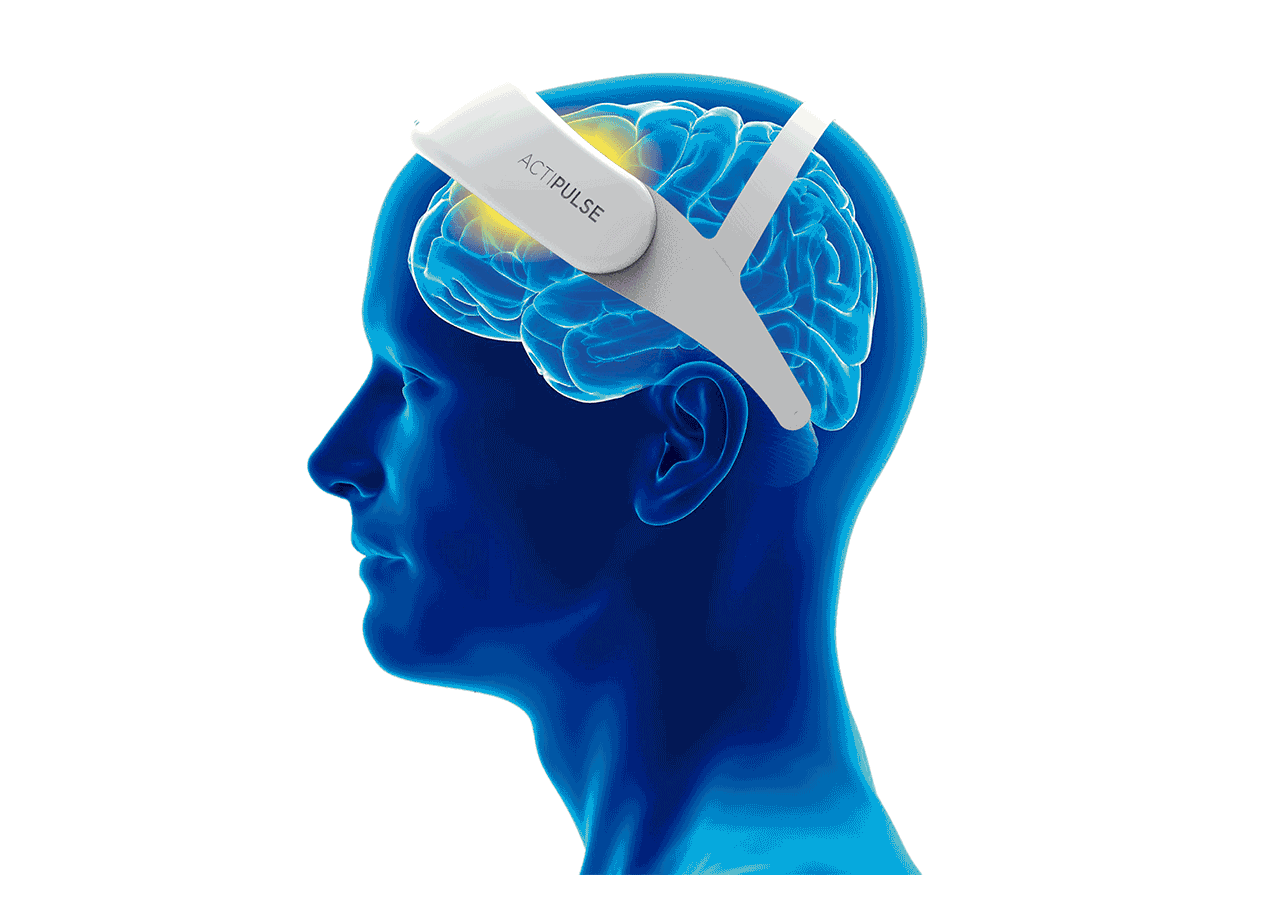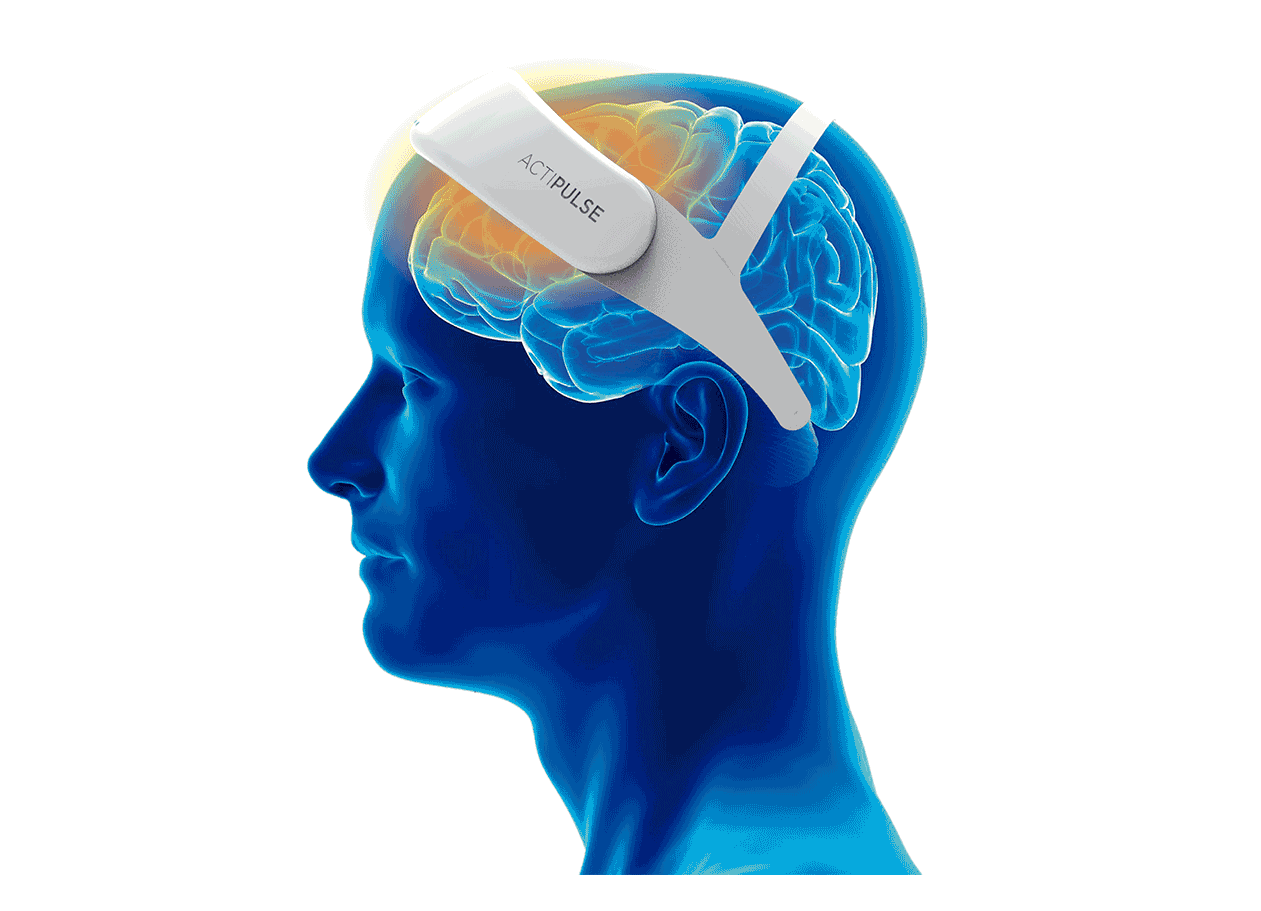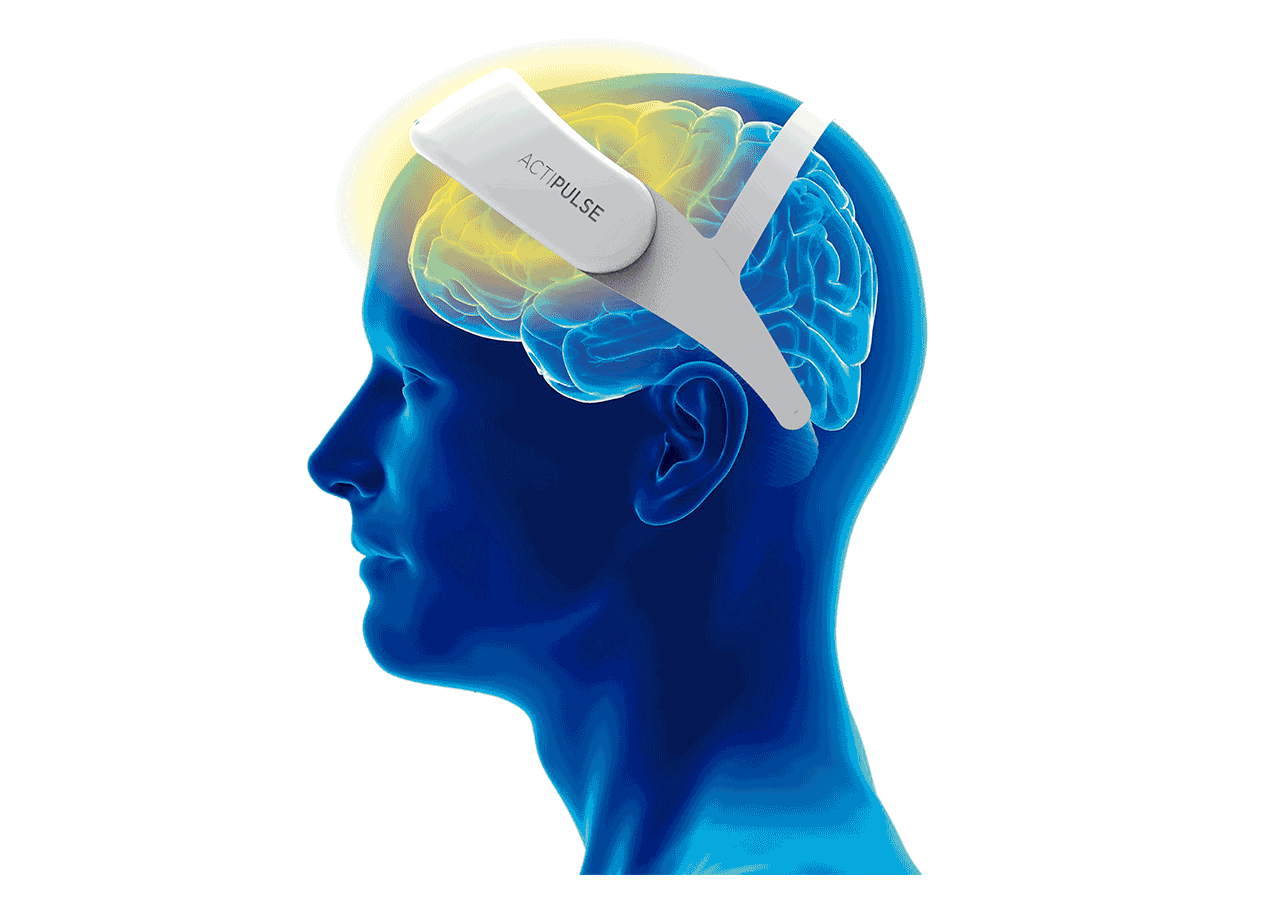
Patients will require 60 sessions of stimulation (twice per day), which they will receive from the comfort of their homes, without the need of a medical professional to apply the therapy.
Patients can do any type of activity during their 45 minutes treatment (reading, TV, etc), and they remove the device after each session.
Their physician will have access to the patient's data to monitor use, and any side effects that the patient can report via the connect app.
Traction
A clear path to FDA clearance
We have already filed our FDA pre-submission (May 2021) in order to receive the FDA’s consent for the protocol design that will be used for the pivotal clinical trial.
We are aiming to begin patient recruitment for the randomized, double-blind, and controlled trial by January 2022, and to complete it by October 2022.
Our goal is to receive FDA clearance in 2023.
Customers
Actipulse has helped 10K+ patients through June 2021
Thanks to our 210+ hospital-setting neuromodulation devices currently in use in psychiatric practices and mental health clinics, we have managed to treat more than 10,000 patients as of June 2021.
Our goal is to treat a 100,000 patients by 2025. With your trust, we will achieve this objective.

Business model
Our hospital-setting devices generate $30,000 per end-user and per year, on average
Our business model has two modalities: patient direct use with our at home device (post FDA approval) and shared-revenue for our hospital-setting device (currently on market).
We anticipate that the Actipulse Home device revenue stream will rapidly overtake the Actipulse hospital version once it is approved and on the market. It will use a monthly subscription model, where patients will be able to order online the Actipulse Home device directly to their homes, and pay for the duration of their treatment.
It is too soon to determine the final cost of unit economics and cost to treat, as those will be influenced by the pivotal clinical trial results, reimbursement strategy, and competition, once we launch the device in the US market, after FDA approval (Q3 2023).
We currently anticipate that treatment with Actipulse Home will cost between $1,200 and $1,600 for an anticipated 3 month duration. This is best compared to neuromodulation treatment in a hospital setting that costs an average of $15,000.
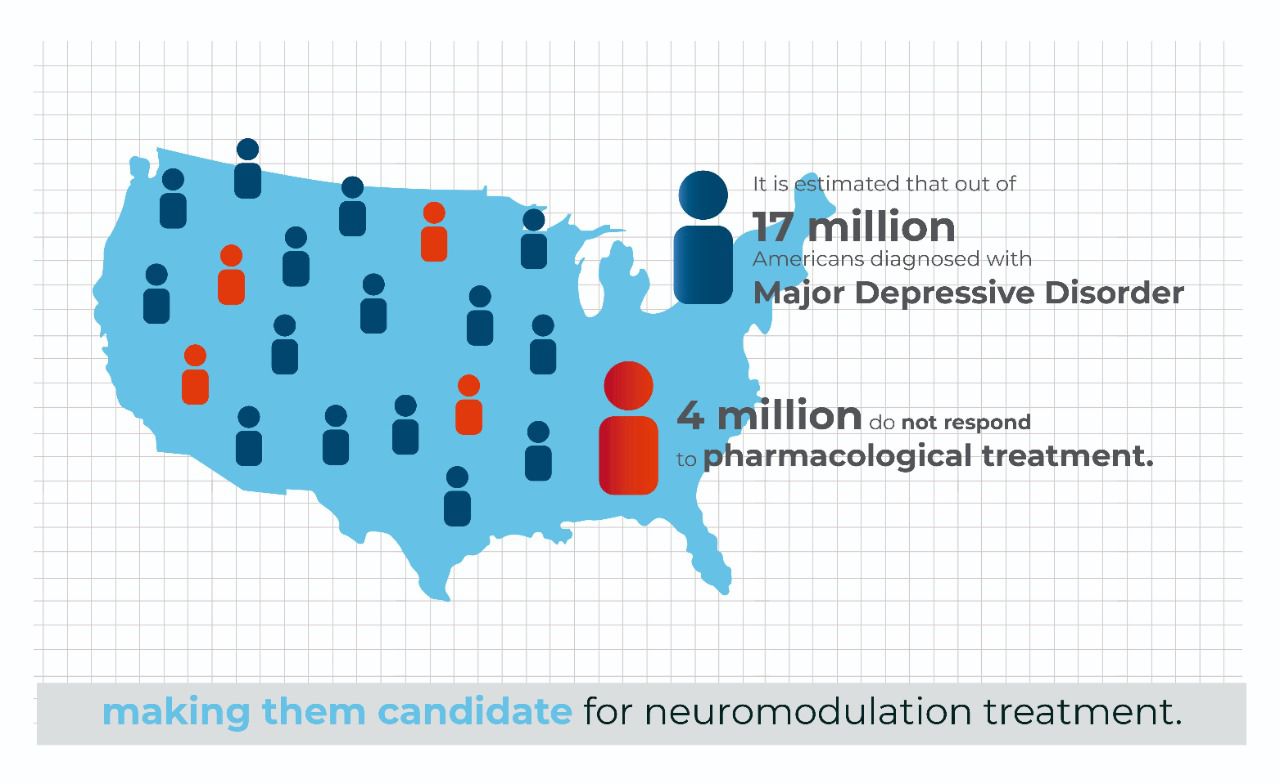
It is estimated that out of 17 million Americans diagnosed with Major Depressive Disorder, 4 million do not respond to pharmacological treatment, making them candidates for neuromodulation treatment.
For illustration purposes, if we chose a market penetration pricing of $1200/ treatment, this would equal a $4.8B addressable market, with this number greatly expanding, if we chose a higher cost of treatment with a reimbursement pathway.
Market
A $4B market for neuromodulation treatment for MDD in 2020
Actipulse operates in a growing market dedicated to the treatment of mental illnesses. We project that we will reach $7M+ in revenue by 2023. We base this forecast on our historical revenue growth, innovation based on market demand, and the growing brand awareness of Actipulse in Latin America.
Competition
Actipulse competes in the brain neuromodulation market
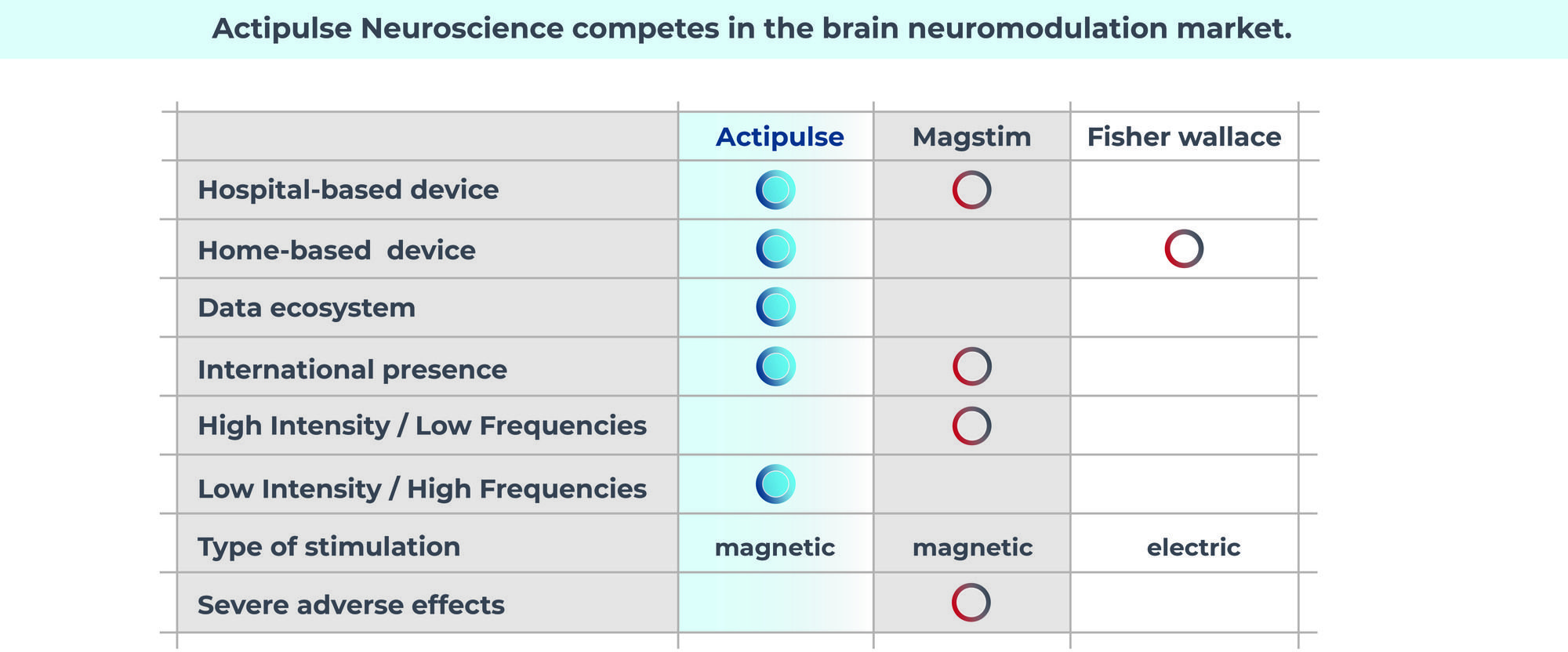
Treatment for the mental illness market is vast and undominated. There are a few players for home treatment, but most are based in the wellness, at not the medical market.
Traditional medications are not able to treat everyone and are frequently used in conjunction with non-invasive practices like Actipulse.
Vision and strategy
The leading medical neuro-technology company by 2025

Our goal is not to become a $1 billion dollar company,
but a "1 billion patient company", as we know that the financial success of Actipulse, its investors and founders, comes by putting the patient at the center of our mission.
The latest scientific literature and our current clinical results are showing that the future of neurological and psychiatric treatments will be a combination of pharmacological drugs targeting defined mechanisms such as peptide deposition, excitotoxicity, and metabolic targets, and neuromodulation therapy treating loss of plasticity, loss of neurogenesis and inflammation.
Our goal is to become the golden standard of clinically proved neuromodulation therapeutics in the next 5 years, and to become an integral part of the treatment of the millions of patients suffering from psychiatric and neurological diseases, in a hospital or home setting.
Founded by an international team, we aim to expand the company beyond the US and the LATAM markets to answer our global ambition. Wherever there is a patient in need, we will be there.
Funding
High-growth, cost-effective medical startup
Actipulse is proud to be a financially responsible medical startup that first achieved profitability in 2019, with only $140K in seed funding, as we know that responsible financial management is key to achieving our long term objectives.
Alumni of prestigious accelerators
Founders
 Adrien Châtillon (CEO) is a French-European serial entrepreneur, Actipulse is his third startup. Passionate about leveraging entrepreneurship to bring solutions to a major challenge that affects the lives of millions of people in the world: access to better and more affordable mental healthcare.
Adrien Châtillon (CEO) is a French-European serial entrepreneur, Actipulse is his third startup. Passionate about leveraging entrepreneurship to bring solutions to a major challenge that affects the lives of millions of people in the world: access to better and more affordable mental healthcare.
Adrien is a polyglot and has lived and worked in more than seven countries. When not working, you can find Adrien daydreaming about becoming an F1driver. It's never too late, according to him.
Gabriel Villafuerte (CMO) is a Mexican scientist. He is a licensed Medical Doctor, and holds a PhD in Neuroscience. His research focuses on using neuromodulation to treat neurodegenerative diseases in order to reduce cognitive and motor symptoms linked to these terrible pathologies.
Villafuerte (CMO) is a Mexican scientist. He is a licensed Medical Doctor, and holds a PhD in Neuroscience. His research focuses on using neuromodulation to treat neurodegenerative diseases in order to reduce cognitive and motor symptoms linked to these terrible pathologies.
His research has been published in prestigious international medical journals.
When not in the lab unlocking the secrets of the human brain, you can find Gabriel practicing his other passion: cooking.
 Daniel Gomez (COO) is a serial entrepreneur with more than 5 ventures to his name. With more than 15 years of experience in launching and managing tech startups, he is Actipulse's chief of operations, making sure the company runs a steady course and meets its quarterly objectives.
Daniel Gomez (COO) is a serial entrepreneur with more than 5 ventures to his name. With more than 15 years of experience in launching and managing tech startups, he is Actipulse's chief of operations, making sure the company runs a steady course and meets its quarterly objectives.
Daniel once stayed 4 weeks alone in the Amazon forest, he says that it was intentional, but some wonder if he got lost. In any case, an incredible story of survival.
The Company currently conducts the majority of its business through its wholly-owned subsidiary, Actipulse International SA de CV, a Mexican corporation, incorporated on April 17, 2017. Please see our Form C for additional disclosures.







 Oops! We couldn’t find any results...
Oops! We couldn’t find any results...

















