Problem
Have you ever taken medicine in the form of a pill?
Medication intake in the form of a pill goes through our digestive tract and takes a long time to get into our bloodstream in order to become effective. This traditional form of intake also causes various issues to our stomach, liver and other organs.
Traditional Aspirin takes about 20 minutes on average to begin to become active in our body, and likely twice that time to reach its full potential effectiveness. It is a great anti-inflammatory and pain reducing pharmaceutical but taken every day as a pill causes severe damage to our stomach and liver.
Solution
Our technology delivers medicine more effectively and safer
Aspire BioPharma's has developed technology that allows for medicine to flow into our bloodstream directly from our mouth instead of going through the digestive tract. This allows for a much faster delivery method with minimal impact on our liver.
Our technology is a novel soluble drug formulation delivering full dosage with minimal impact on stomach and liver, with the potential to change the delivery method of various drugs, delivering Ph neutral, powder formulation developed through its patented formulation, and "trade secret” process.
Aspire's launch product, Instaprin, will be an aspirin formulation which addresses cardiology and stroke emergencies, acute pain management and anti-inflammatory needs.
Product
Instaprin
Initially, Aspire intends to commercialize its novel soluble formulation for aspirin for cardiology and stroke emergencies and pain management. Aspire’s technology utilizes a new mechanism of action (the absorption pathway) which allows for rapid sublingual and high absorption in the mouth. This sublingual delivery method avoids direct damage to mucus secreting goblet cells lining the gastrointestinal tract as well as avoiding bioavailability reduction by first pass hepatic metabolism as seen through alternative delivery methods such as suppository, intramuscular and transdermal.
In the Company’s launch product, the aspirin formulation, this is critical in stopping heart attacks and strokes, and the high dosage formulation is effective for pain management including quick headache relief, post-surgery, cancer pain management, and general pain relief.
Aspire believes that its technology with the potential to be applied to any number of proven, approved, “do no harm” drugs such as analgesics (pain relievers) and, for example, various erectile dysfunction and traumatic brain injury drugs.
Aspire intends to target the analgesics market and plans to launch its first product, Instaprin, for the prescription market in financial year (“FY”) ending December 31, 2025 and the over-the-counter ("OTC") version in FY2024. The Company also sees an opportunity to generate licensing revenues beginning in FY2025 by licensing its proprietary technology to third party pharmaceutical companies for other drugs.
Traction
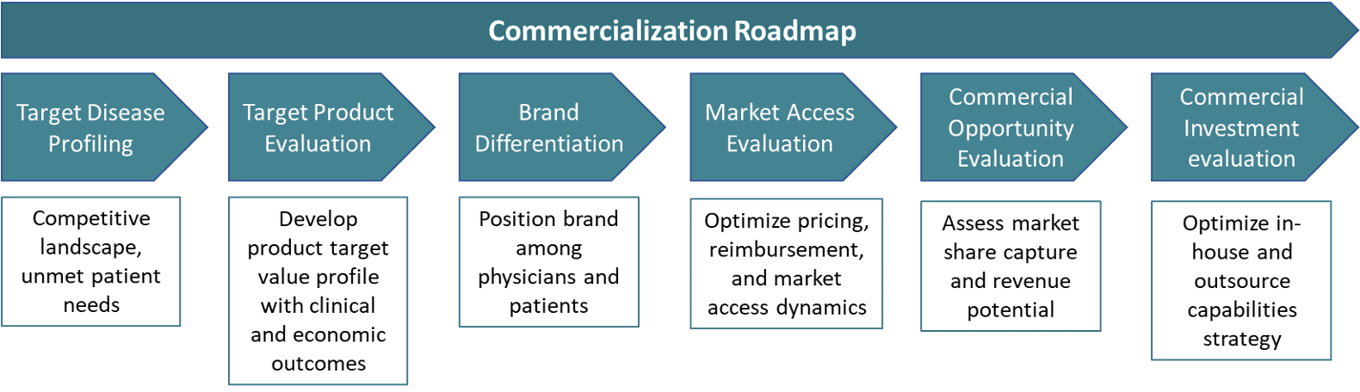
Manufacturing Strategy
A contract manufacturing strategy is being pursued with the potential for multiple supplier relationships.

- Technical Manufacturing Development Partner
- Pharmaceutical Development Agreement signed June 2022
- Engaged to complete a dry blend and wet granulation process feasibility study in July 2022

- Currently in discussions for an alternate outsourced manufacturer
- Discussions include developing a rapid dissolving sublingual tablet form
Employing a contract manufacturing strategy with proven supplier(s) eliminates the risks and capex associated with building and running a manufacturing facility while increasing ROI by avoiding large capital investments, particularly in Aspire’s early years.
Customers
Instaprin: Overview
Instaprin, which addresses cardiology emergencies and pain management, is a granular or powder formulation of a soluble, Ph neutral, fast acting aspirin which has been developed by using our patented formulation, and "trade secret” process.
Benefits of “instant absorption” aspirin are: to stop heart attack and stroke; allow high dose absorption for pain management including quick headache relief, post surgery, cancer pain management, and general pain relief.
Instaprin: Opioid Alternative Opportunity
Opioids, while still broadly prescribed, have become the driver of one of the most significant consequential health crisis of our time, resulting in destructive social, cultural, economic, and ethical impacts.
Instaprin is expected to have the support and tailwinds from regulatory bodies, the medical industry, medical insurance markets, political constituents, as well as the patient population, as an alternative to the troublesome opioid analgesics in the market today.
We are currently in discussions with regulatory bodies to “Fast Track” Instaprin, a “do no harm” aspirin medication through the FDA and regulatory agencies to leverage the issues of the current anti-opioid initiatives.
This environment is expected to enable Aspire to grow rapidly with greatly reduced risk and reduced marketing expense levels relative to most other entrants to the biopharma space who are more likely focused on relatively narrow cure or therapy markets.
Business model
The Company intends to pursue contract manufacturing, marketing, and distribution strategies to leverage existing professional expertise and industry knowledge bases and to eliminate the risks and capital investments associated with building and operating a manufacturing facility.
Aspire has signed a pharmaceutical development agreement with Glatt Air Techniques Inc., a Ramsey, New Jersey based company engaged in the development and manufacture of pharmaceutical products with particular expertise in pharmaceutical galenics, in June 2022.
Market
Instaprin is expected to have the support and tailwinds from regulatory bodies, the medical industry, medical insurance markets, political constituents, as well as the patient population, as an alternative to the troublesome opioid analgesics in the market today.
Aspire BioPharma is currently in discussions with regulatory bodies to “Fast Track” Instaprin, a “do no harm” medication through the FDA and regulatory agencies to leverage the issues of the current anti-opioid initiatives.
This environment is expected to enable Aspire to grow rapidly with greatly reduced risk and reduced marketing expense levels relative to most other entrants to the biopharma space who are more likely focused on a niche market that doesn’t necessarily have the social, cultural, economic, and ethical impacts of the very broadly prescribed opioid analgesic alternative.
Aspire is pursuing various initiatives at different stages ranging from initial discussions to verbal commitments with various retail and distribution partners to commercialize Instaprin and implement the company’s marketing strategy.
Competition
The Company has developed and acquired disruptive technologies that are a Novel Soluble Formulation which address emergencies and drug efficacy, dosage management, and response time.
Patent protected technologies are shielded from competition and may generate significant revenue growth and earn a high margin during the patent protection period.
Aspire BioPharma has acquired the intellectual propery for the formulation and technology that underlies the original Instaprin patent (Patent No. 62/794141 filed with the USPTO on January 18, 2019, and International Application Number PCT/US2020/014218 filed with the World Intellectual Property Organization under the Patent Cooperation Treaty on January 18, 2020).
In March 2023, the Company filed application number 63/456,290 with the United States Patent and Trademark Office (“USPTO”) with the goal of securing patent protection for its new technology and aspirin formulation. The Company’s new patent pending formulation is a significant improvement on the previously patented formulation which was acquired by the Company through the Instaprin Pharmaceuticals, Inc. acquisition (described below). This technology is expected to facilitate development of any number of products in a soluble, PH neutral, fast acting powder form which has been developed by using our patented formulation, and "trade secret” process. Aspire’s drug delivery comes from a new mechanism of action (absorption pathway) which allows for instant absorption in the mouth. The benefits of “instant absorption” are to provide nearly instant treatment impact and also allows high dose absorption. The Company’s patented and patent pending delivery system includes components specifically formulated to allow rapid sublingual absorption of drugs into the blood stream, thus by-passing the gastrointestinal tract.
In the initial launch of its “Instaprin” product, Aspire has focused on the delivery of aspirin, which may be the most studied and accepted analgesic and anti-inflammatory. Aspirin is over a century old and is traditionally available in several forms, effervescence, powder and tablet. Over 100 years of documented safety and efficacy data is readily available. Aspirin is the only drug in history to receive a certified recommendation by the FDA for heart attack, stroke and colon cancer. However, current aspirin applications are limited due to side effects from acidity. Instaprin has no acidic side effects, nor is it metabolized through the liver. We further expect that Instaprin will be well positioned to target the current Opioid Crisis globally due to its ability to have large doses rapidly be absorbed in the bloodstream with no harmful effects to the gastric system and its mucous membrane, as well as, at full strength with no dilution due to metabolic impact, providing true anti-inflammatory therapeutic effects to users and providing true pain management relief to them.
Instaprin Regulatory Status. The Company plans to rapidly file Instaprin to be OTC FDA monograph compliant. Additionally, Aspire plans to seek FDA 505(b)(2) Fast Track designation for the prescription strength Instaprin product by end of Q2 of 2024.
Vision and strategy
Aspire BioPharma intends to bring to market Instaprin as the leading product that will demonstrate our unique technology. After a successful launch and adoption, the company plans to expand its revenue stream by licensing its technology to larger pharmaceutical companies that seek alternative and more effective absorbing of their products.
Aspire BioPharma delivers an innovative product by showcasing its patent protected technology and aims to bring innovation to the entire pharmaceutical industry.
With Instaprin, our main motivation is to address a patient's critical pain and inflammation conditions, save victims who suffer from the acute risks of heart attack or stroke, and to address the rapidly growing Opioid Crisis in North America and around the world.
Impact
Analgesics, commonly referred to as painkillers or pain relievers, are medications that reduce pain without disturbing consciousness, altering sensory perception, or interrupting the conduction of nerve impulses. These medicines include acetaminophen, often known as paracetamol or APAP in North America, salicylates, a class of nonsteroidal anti-inflammatory medications (“NSAIDs”), and opioids including morphine and oxycodone. Physiological injuries, operations, phantom pains, inflammation, neuropathic diseases, and cancer therapies all cause pain, which is treated with analgesics.
The strongest painkillers come in the form of opioid drugs, which are made from opium. They are often used to treat moderate to severe pain and are only accessible with a prescription. Contrarily, acetaminophen is used to make non-opioid medications rather than opium. Another group of medications called NSAIDs (non-steroidal anti-inflammatory drugs) is frequently used to alleviate mild to moderate pain.
According to a research report by Precedence Research, as shown in the below chart, the global analgesics market including opioids was valued at $105.8 billion in 2021 and it is expected to reach around $149.3 billion by 2030 growing at a compound annual growth rate (“CAGR”) of 3.9% during the forecast period from 2022 to 2030. North America region accounted for 42.3% revenue share in 2021.
Funding
Aspire BioPharma has raised capital from its founders, key executives, family and friends, accredited investors and is supplementing its capital raise via crowdfunding.
Our founders and large key investors will continue to support Aspire BioPharma with additional capital as needed as we gear up for regulatory approvals and market launch of Instaprin.
Founders
Aspire BioPharma’s management team has decades of combined corporate business experience. Notably, Kraig Higginson, Principal, brings to the Company extensive experience in early-stage growth company management as well as vast public market expertise.
Mr. Higginson served as Chief Executive Officer of VIA Motors, Inc. (“Via Motors”), a hybrid electric vehicle company (PHEV), from November 2010 to January 2014, where he was responsible for overseeing the management and business of Via Motors and its employees. From October 2003 until November 2010, he served as Chairman of the Board of Directors of Raser Technologies, Inc. (“Raser Technologies”), which was an NYSE listed company at that time. Mr. Higginson also founded American Telemedia Network, Inc. (“American Telemedia”), a publicly traded NASDAQ company that developed a nationwide satellite network broadcasting data, video programming and advertising to shopping centers and malls, and he served as President and Chief Executive Officer of American Telemedia from 1984 through 1988. Mr. Higginson’s years of experience in the management of public companies is a great asset to the Company.
Key Executives
Ernest Scheidemann, Jr., Chief Financial Officer (“CFO”), brings over 20 years of CFO experience leading financial, operational and administrative aspects of both public and private companies, in Fortune 500, mid-cap, mega start-ups and fast-paced entrepreneurial organizations in a variety of industries, and has served on boards of health care services related non-profits.
Stephen Quesenberry was appointed General Counsel and Corporate Secretary of Aspire Biopharma, Inc. in September, 2021. Mr. Quesenberry has practiced law since 1989 in Washington and Utah, including complex business litigation and SEC matters. Mr. Quesenberry was one of the (many) attorneys representing Exxon Shipping in the Exxon Valdez litigation in Alaska in the early 1990s.
Lance Friedman, Director of Investor Relations - Mr. Friedman began his career in the legal field with a focus in corporate, securities and merger and acquisition transactions, before transitioning to merchant/Investment Banking and business operations in 1987. In the mid-90s, he was hired by healthcare and real estate magnates Abraham Gosman and Bernard Marden to become a part of senior management in various investment led healthcare focused companies, including WirelessMD and WebMD. Subsequently, Mr. Friedman was invited to join EGL Holdings as Managing Director/Partner, a well-known cross border venture capital and boutique investment banking firm in 2003 to manage the healthcare investment and advisory practice, including serving as an internal senior level capital markets and M&A member of VertiSoft resulting in its sale to Optio Software. He also served as COO and EVP of Finance of Fletcher-Flora Health Care Systems, Inc., resulting in the sale of the company to Merge Healthcare. Recent appointments include CEO of First Choice Healthcare Solutions, Inc., and formerly of Instaprin Pharmaceuticals, Inc to execute an exit due to an SEC matter consent judgment issued to the Company and its founder, and VIA Motors International leading its capital markets strategy for a period of 8 years culminating in a merger valued at $630M.
Medical Advisory Board
James Kofi Dzandu, Ph.D, Dr. Dzandu received his Doctor of Philosophy Degree in Biochemistry from Wayne State University, Detroit Michigan in 1980. He was appointed Assistant Professor of Pathology and Associate Director of the DNA/Identity laboratory at University of Texas Health Science center, Fort Worth Texas. Dr. Dzandu is well published in peer-reviewed journals and has received many awards and funding support for his work on sickle cell, proteomics and genomics. In the last few years, Dr. Dzandu has turned his attention to a successful collaborative research partnership with Dr. Mangram and the trauma dream team on trauma research with special emphasis on G60.
Morhaf Ibrahim, MD, FHRS, FACC, As an Electrophysiologist and Cardiologist, Dr. Morhaf Ibrahim is passionate about all aspects of cardiology. He completed his fellowship in cardiac electrophysiology at the University of Florida Jacksonville and Mayo Clinic Jacksonville. He is excited to connect with his patients and make a significant impact in each of their lives. He brings with him a commitment and experience to treat not only cardiac arrhythmias but other aspects of heart diseases as well. Dr. Ibrahim graduated from the University of Damascus in Syria. He then completed his residency in Houston at the University of Texas, Texas Medical Center. He was fascinated about the field of cardiology and decided to complete his training in Cardiology at the University of South Alabama where he served as a chief cardiology fellow. During his cardiology training, he became interested in cardiac electrophysiology and he came to Jacksonville where he was trained in Cardiac Electrophysiology.
Gary Bernard, MD, Internal Medicine Specialist has over 30 years of experience in the medical field. He graduated from Meharry Med Coll in 1992. He is affiliated with HCA Florida Orange Park Hospital
Paul Montanarella, MD, Chief Anesthesiology, Honor Health Hospital System earned his medical degree from the Universidad Nacional Autónoma de Honduras Facultad de Ciencias Médicas. Upon graduating, he relocated to the United States and completed his residency in internal medicine at the Highland Hospital of Rochester in 1988, and his residency in anesthesiology at Westchester Medical Center and SUNY Upstate Medical University in 1991. With an unwavering commitment to his specialty, the doctor is board-certified in anesthesiology by the American Board of Anesthesiology (ABA). As the certifying body for anesthesiologists since 1938, the ABA is committed to partnering with physicians to advance the lifelong learning and exceptional patient care. Its mission is to advance the highest standards of the practice of anesthesiology. Anesthesiology is the medical specialty concerned with the total perioperative care of patients before, during, and after surgery. It encompasses anesthesia, intensive care medicine, critical emergency medicine, and pain medicine. Anesthesiologists have the primary responsibility of monitoring the patient’s vital signs during surgery. In addition to basic measurements such as pulse, blood pressure, and temperature, they measure the patient’s respiration.
Edward Kimball, MD, Dr Edward Kimball is a Professor of Surgery at the University of Utah Health Sciences Center and Medical Director of Surgical Critical Care at the Salt Lake VA Medical Center. He is the Chief Medical Officer for Outreach Network Development and Telehealth and Medical Director of TeleICU services for U Health. Dr. Kimball’s research in critical care medicine has been focused on shock resuscitation, inflammation and its effects on abdominal organ function. He and his colleagues designed the device used as an international standard for assessing intra-abdominal pressures in critically ill patients. He is the current president of the World Abdominal Compartment Society. Dr. Kimball served as a medical officer in the US Army and continues to provide training for US Special Forces. He is married to Rebekah Ellsworth Kimball, has four children and resides in Salt Lake City.
Gary Bernard, MD - President and CEO, Pointe Medical Services, Inc., President and CEO, Pointe Med Pharmacy, Inc., Owner Live, Well MD, LLC, Majority Owner and Managing Member, Live Well Drugstore, LLC, d/b/a TruLife Pharmacy. Dr. Bernard completed Residency in Internal Medicine at the University of Florida Health Science Center, Residency in Internal Medicine, Rotation in Cardiology at the Brown University School of Medicine, and Residency in Anesthesiology At the Dartmouth-Hitchcock Medical Center. He earned a B.S. Degree in Chemistry from Hobart and William Smith College, and medical degrees from the University of South Florida and Meharry Medical College.
John A. Mansour Jr, DO - Dr. Mansour is a board certified and fellowship trained orthopaedic surgeon specializing in orthopaedic traumatology and fracture care. He is Chief of Orthopaedic Surgery as well as Medical Director of Orthopaedic Trauma for The Hughston Clinic at HCA Florida Orange Park Hospital. He earned a Bachelor of Science degree in biological sciences, graduating summa cum laude, from Delta State University. He then went on to earn his medical degree in osteopathic medicine from Nova Southeastern University. After finishing his internship at the Peninsula/NS-LIJ Hospital Consortium, he completed his orthopaedic surgery residency at the Philadelphia College of Osteopathic Medicine and continued his training through an orthopaedic traumatology fellowship at Grant Medical Center.
Strategic Advisors
Nancy Taylor, FDA Advisor, Chair of the Health Care & FDA Practice and focuses her practice on health and FDA related matters
Michael Shuster, Partner, Goodwin’s Life Sciences group, providing strategic intellectual property advice to biotechnology, chemical, pharmaceutical, and other life sciences companies
Arthur Marcus, Legal/Securities, Sichenzia Ross Ference LLP, with a practice focus on corporate, commercial, and securities law
Miriam Walls FDA Advisor, X-FDA, works with companies to devise a strategic planning, new opportunity creation, and strategies to help the company remain competitive in the marketplace
Summary
Aspire BioPharma brings innovation to an industry that has an impact on the health of almost every person on our planet.
With its pilot product Instaprin, the company will address a market with a vast reach and bring about a much needed positive change.
We strive to have a meaningful and rapid impact in the fight against the opioid crisis by utilizing our first product and follow through with other products or license our technology to larger companies.






 Oops! We couldn’t find any results...
Oops! We couldn’t find any results...
















