Littleton, CO, November 18, 2022 --( PR.com)-- Securisyn Medical awarded its second Advanced Industries Early Stage Capit...
Opportunity
Securing breakthroughs for a nearly $5B+ problem
Practitioners are stuck. If they want to secure a patient's life-sustaining breathing tube, they only have two choices: tape, which is time-consuming and often fails; or tube holders, which just don't hold up against real-world external forces.
All of that is about to change. With leading partners like the SunMed Group Holdings, Premier, Inc., Mayo Clinic, Global Medical Response, and the Department of Defense, we've invented disruptive solutions that upgrade the industry to a higher standard of securement to minimize preventable harm and deaths, while lowering the total cost of care. These innovations comprise a portfolio of first-of-their-kind interlocking securement systems.

Problem l
Current breathing tube securement is costing lives
In health care, a significant threat to ventilated patient safety is unplanned extubation, which occurs when a patient or other external force pulls an inadequately stabilized breathing tube out of the airway.
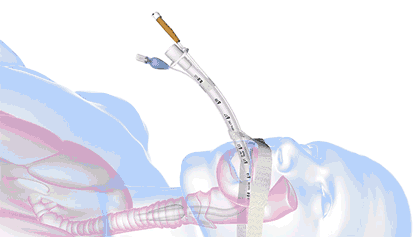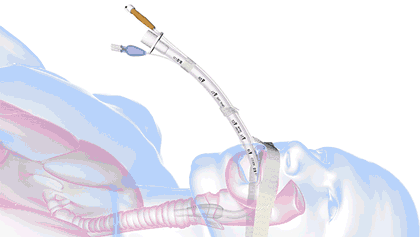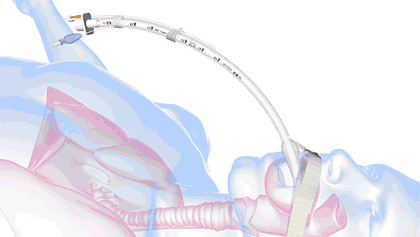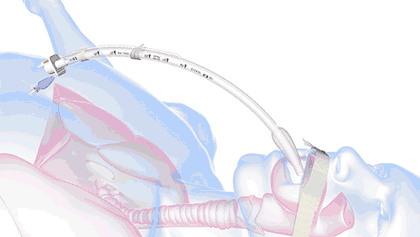
It's a common and costly problem, occurring in 7% of adults, 8% of pediatric patients, and 18% of neonatal patients—and worse, it is also associated with 33,000 preventable deaths annually.
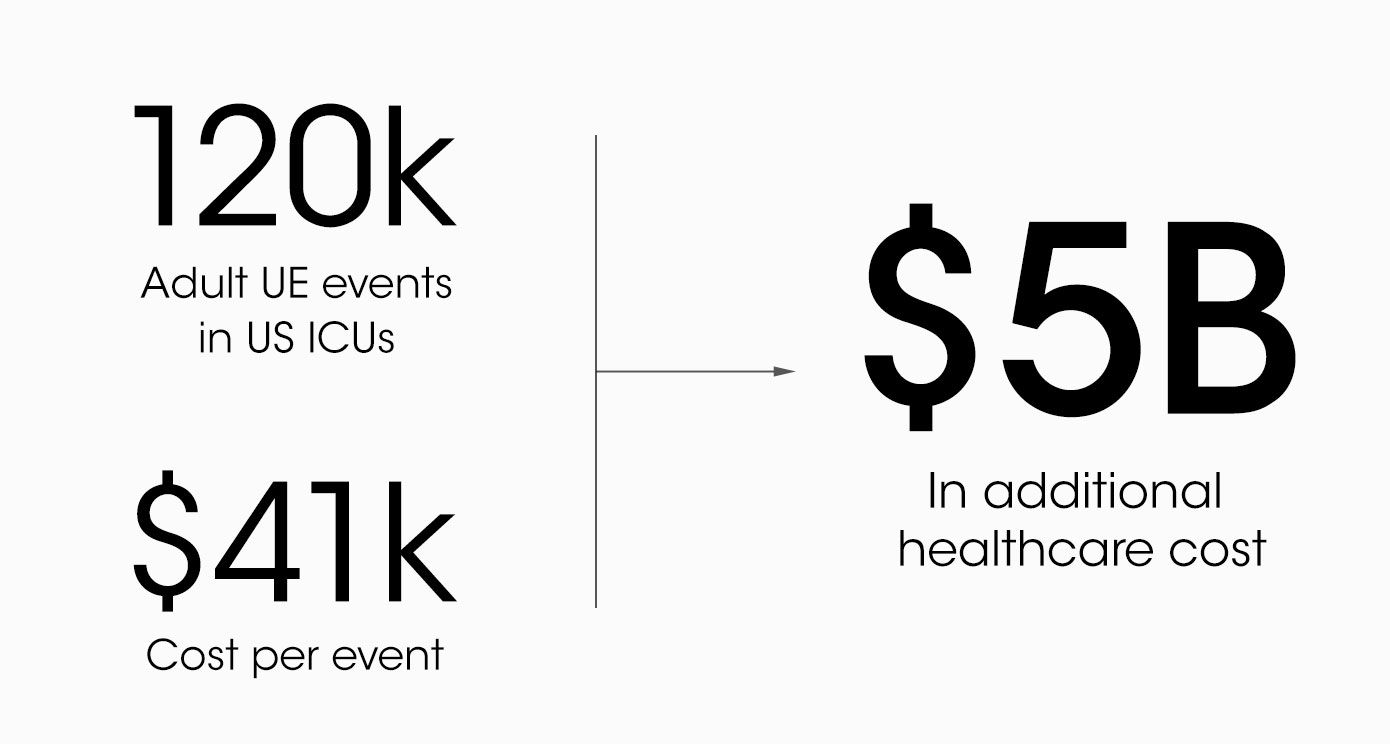
18.8M breathing tubes are placed every year in the US alone for surgeries, injuries, and illnesses like COVID. For each of those, adhesive tape, twill tie, or a commercial tube-holder device secure the breathing tube to the patient.
The lack of adequate securement devices makes it difficult for clinicians to secure breathing tubes during emergencies and in controlled environments, like operating rooms and intensive care units.

The healthcare industry needs a device that can both properly secure and optimally position breathing tubes to avoid serious complications—such as ventilation of only one lung leading to lung collapse, pneumonia, vocal cord injury, and brain injury or death.
COVID increases risk of unplanned extubation
Large numbers of COVID-19 patients have required breathing tubes and ventilation due to severe respiratory disease—struggling to breathe and becoming very restless. When an agitated COVID patient suddenly removes their breathing tube, the virus is spread through the air, putting frontline healthcare workers in harm's way.
Tragically, in the wake of this worldwide pandemic, more people have had first-hand experience with loved ones who needed a breathing tube to keep them alive—and now better understand the importance of effectively securing a breathing tube.
The human costs of inadequate securement

This is Drew. He tragically lost his life at 13—not from the minor head injury he experienced, but from a preventable complication of having his airway managed. Leading to his breathing tube being accidentally removed. Unfortunately, Drew's story isn’t unique and plays out daily in healthcare.
Solution l
An interlocking ribbed collar that secures smooth tubes in place

Our core-enabling technology is revolutionizing the way to secure and stabilize smooth medical tubes.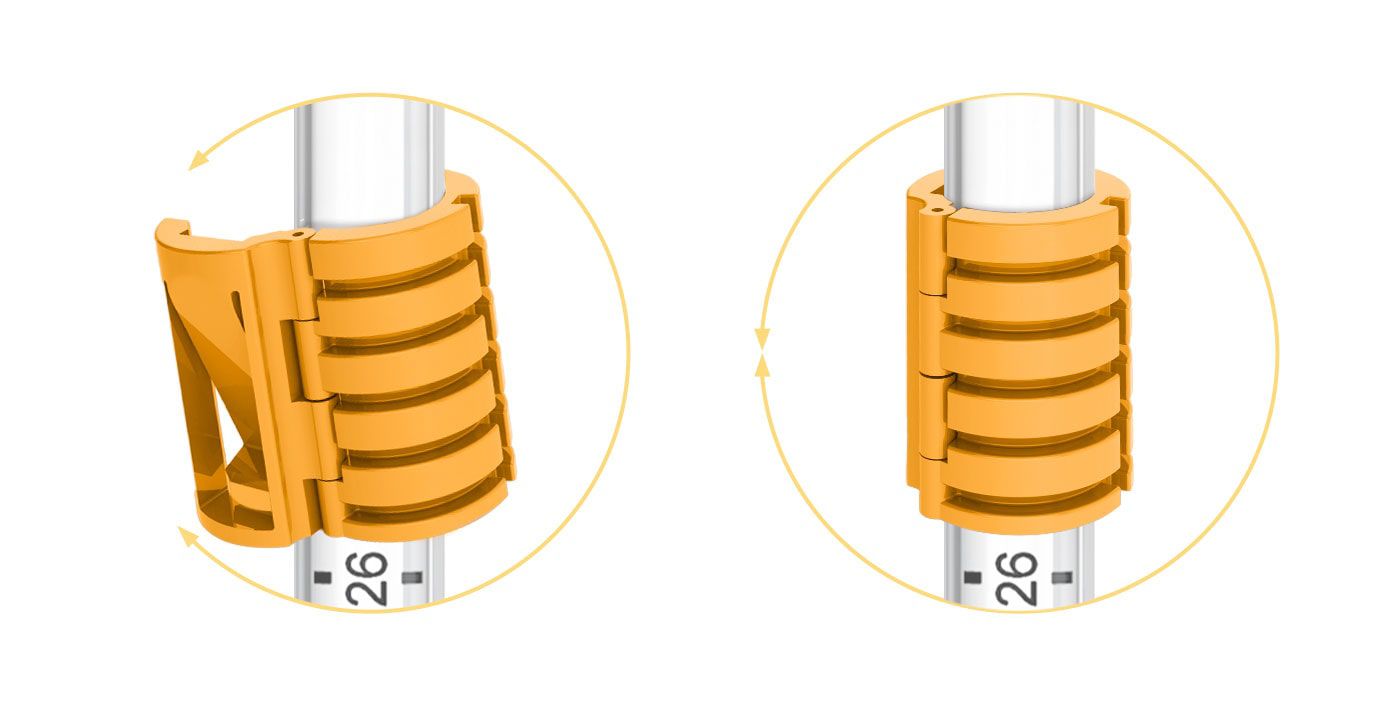 Interlock™ is the patented technology for our breathing tube-securement family of products. Interlock™ is designed with a unique ribbed interface that bonds to commercially available breathing tubes for unmatched strength, improved durability, and fast, easy adjustments.
Interlock™ is the patented technology for our breathing tube-securement family of products. Interlock™ is designed with a unique ribbed interface that bonds to commercially available breathing tubes for unmatched strength, improved durability, and fast, easy adjustments.
Unlike other securement options, Interlock™ eliminates over-constriction of the endotracheal tube’s inner diameter that can lead to impediment of air flow and increased work of breathing.
—
The result is unrivaled securement that outperforms tape and other leading commercial devices.
—
Product l
The new forces in ventilated patient safety
Our portfolio of novel securement devices will change the way the world cares for patients. The portfolio offers exceptional revenue growth with 70+% gross profit potential.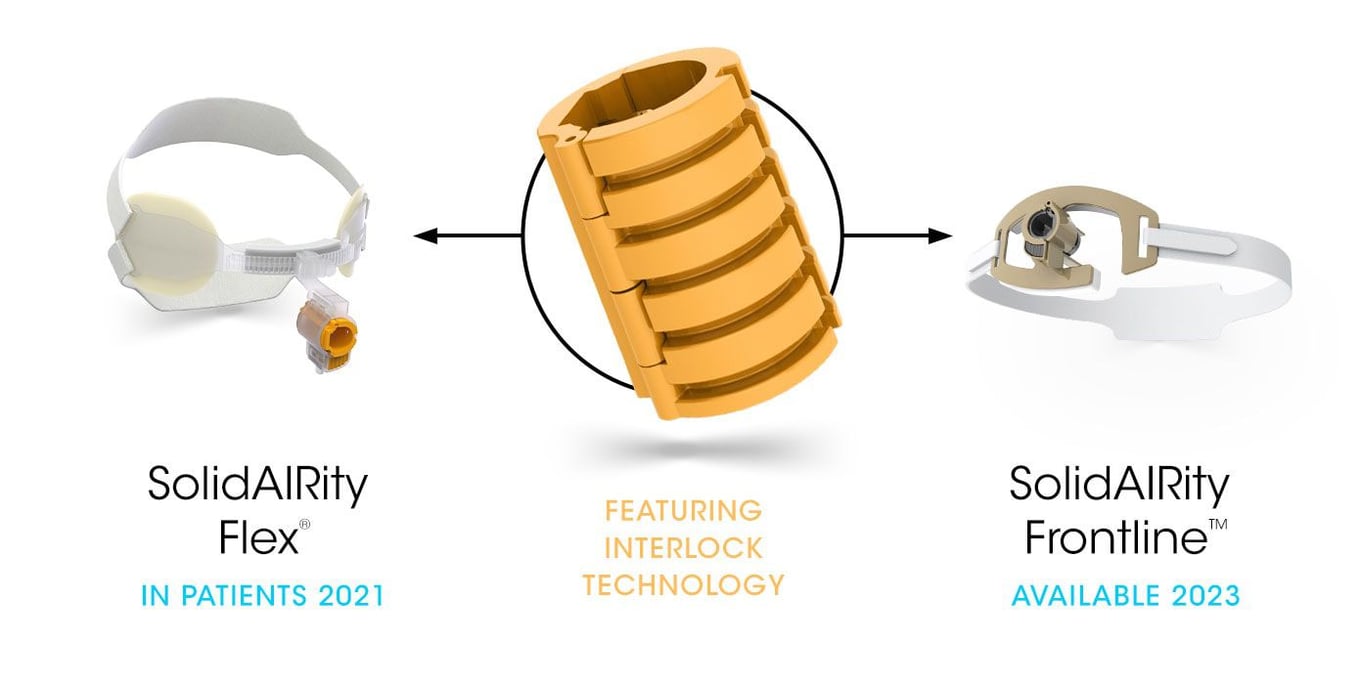

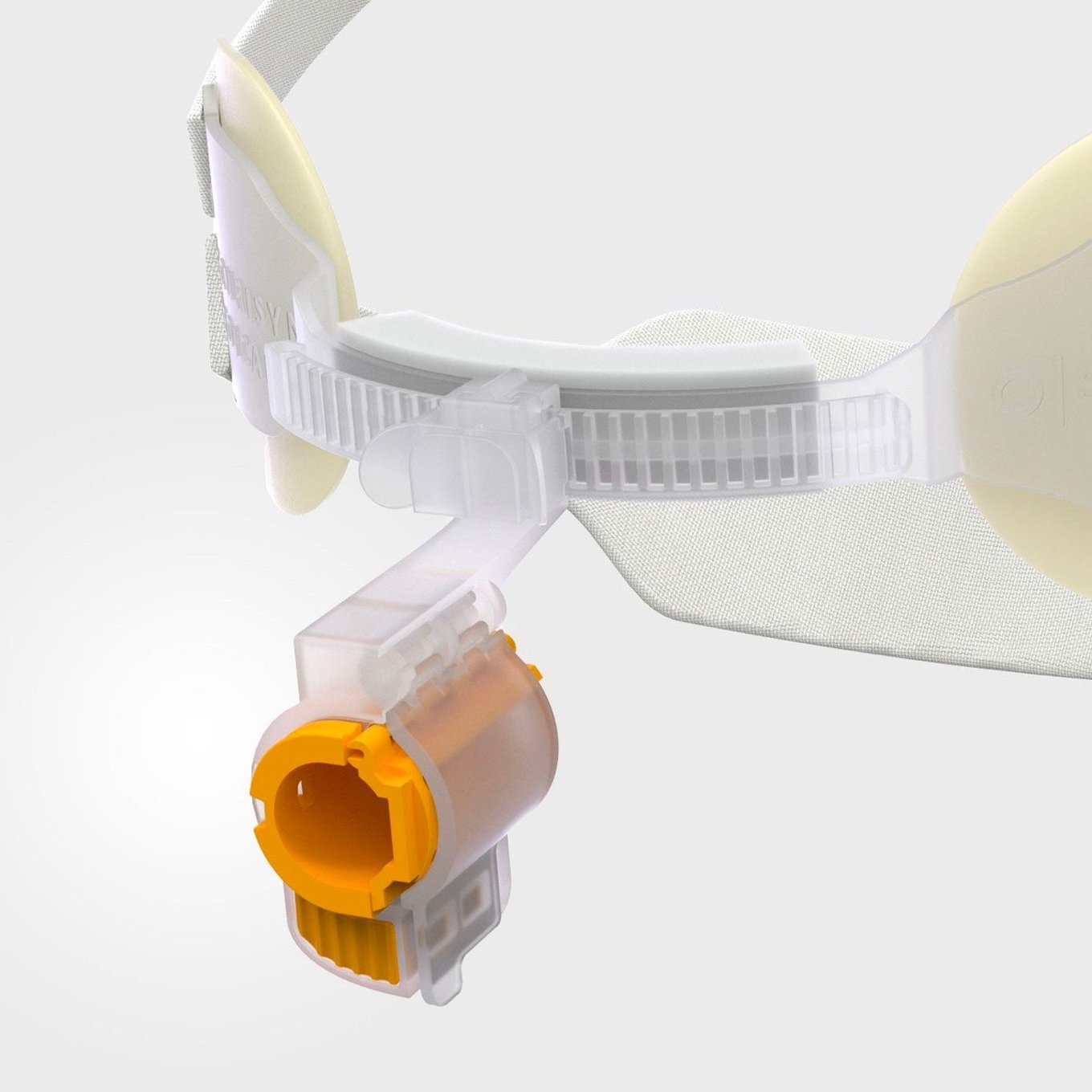
Our integrated endotracheal tube securement device is designed and patented to provide unmatched airway stability for ventilated patients.
What makes this possible is our patent-pending Interlock™ Securement System, a new innovation that provides strong, reliable tube securement against forces that can lead to tube dislodgement and unplanned extubation.


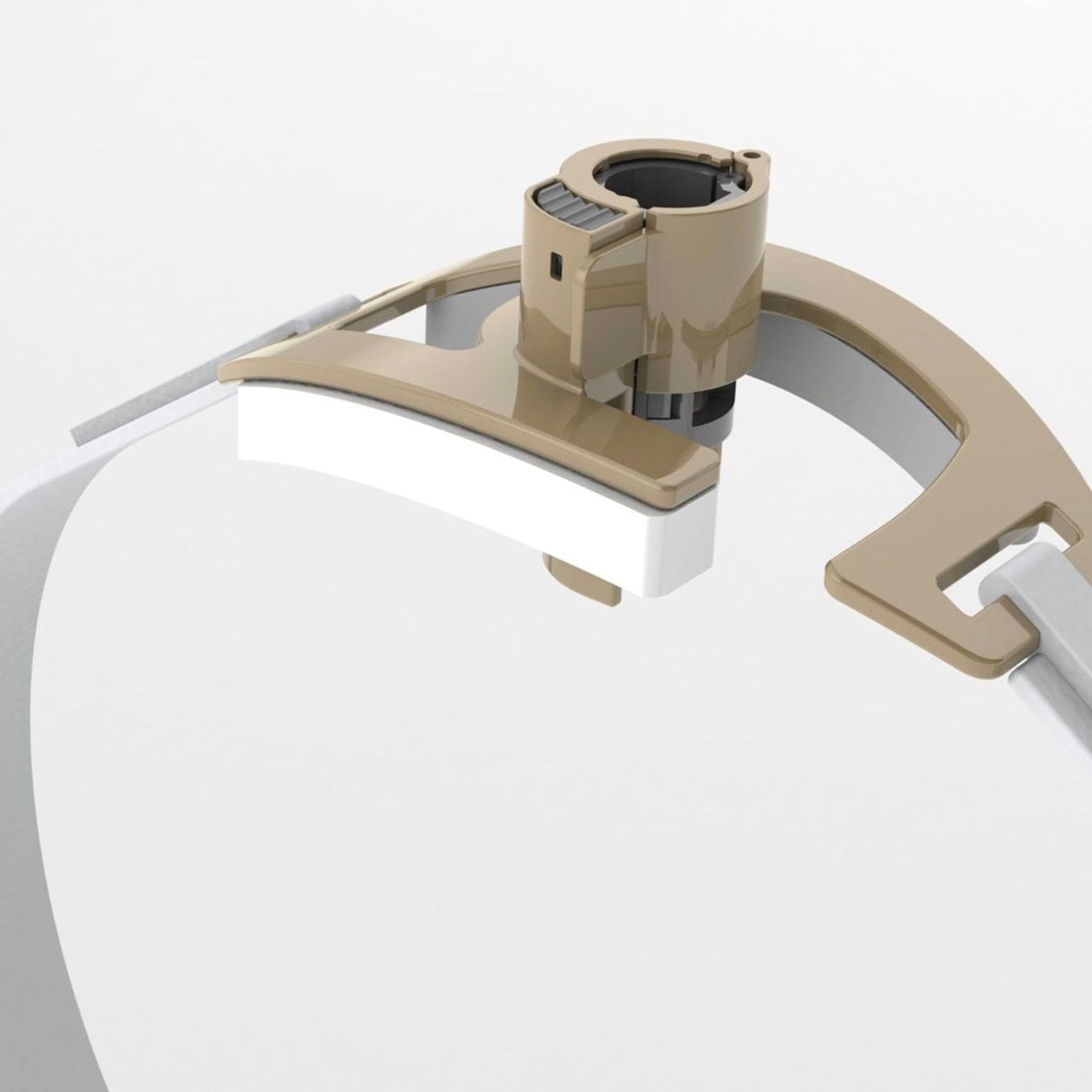
In the austere conditions of combat casualty care, if you don’t have an airway, you don’t have a patient. A single unplanned extubation leading to the preventable death of one Soldier, Sailor, Airman, Marine, or Guardian is one too many.
That’s why we've partnered with the United States Air Force to develop a ruggedized breathing tube securement device that’s battlefield- and civilian EMS-ready.


Coming soon
 Our smallest patients often suffer the most, which is why we've teamed up with world-renowned partners to bring our disruptive breathing tube securement technology to children and premature babies.
Our smallest patients often suffer the most, which is why we've teamed up with world-renowned partners to bring our disruptive breathing tube securement technology to children and premature babies.
Securisyn® and its clinical partners were recently awarded a $1.95M Fast-Track NIH Innovation Research grant to complete our novel pediatric and neonatal breathing tube securement products, specifically designed to reduce unplanned extubation risk for the estimated 130K (US) mechanically-ventilated children.
Our combined technologies have the ability to become the universal smooth tube securement solution and secure other devices like...
Problem ll
Smooth medical tubes & catheters slip too
It's not just breathing tubes. Many medical tubes and catheters are not adequately secured by today’s standards of care. Chest tubes are commonly placed to drain air, blood, or other fluids. More than 1M patients are treated with a chest tube annually.
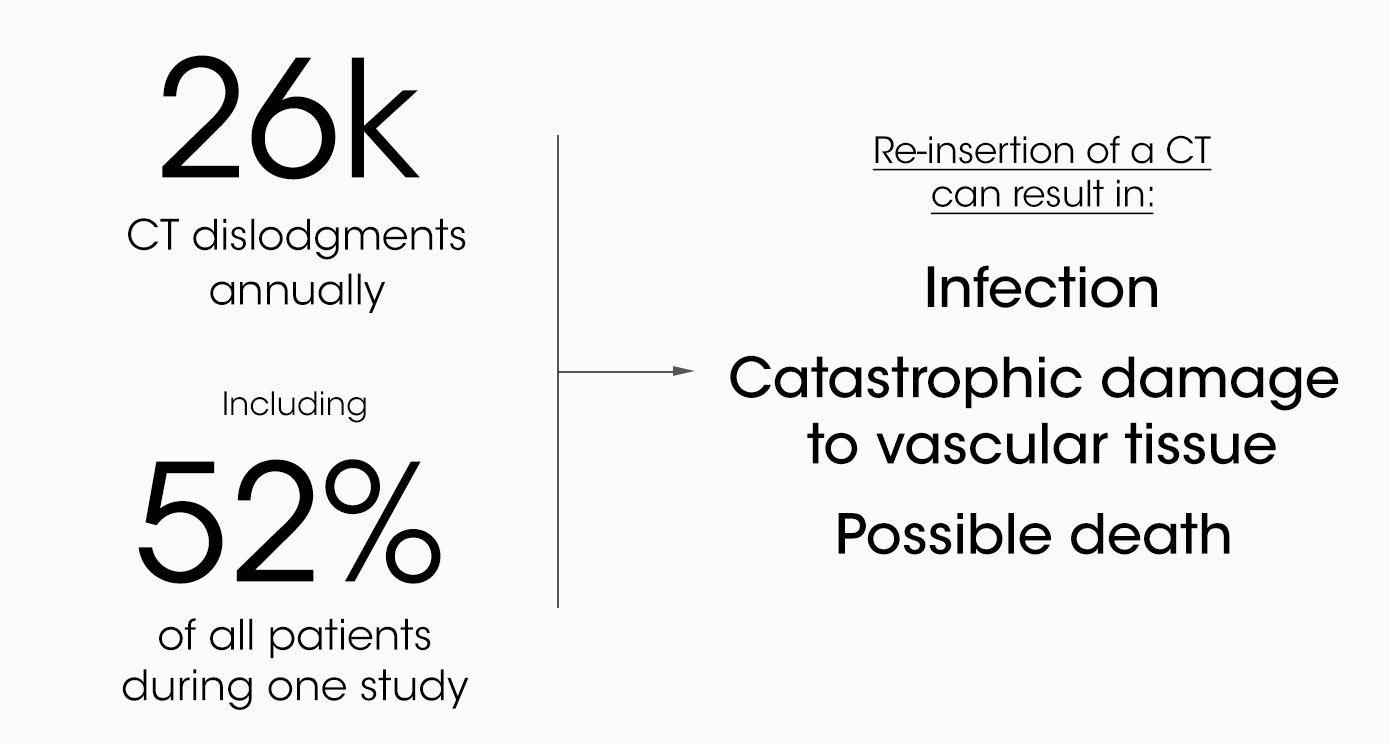
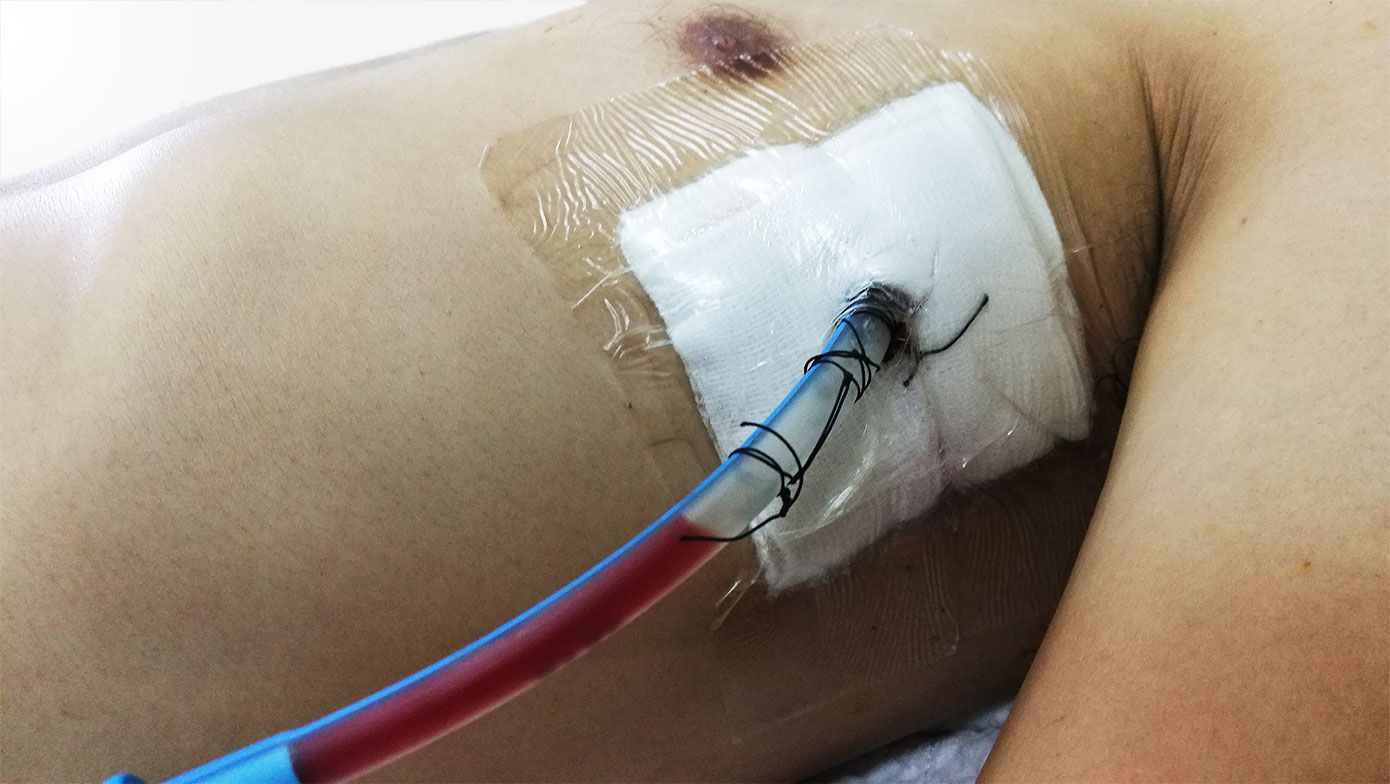
Today, chest tubes are commonly secured with suture and tape or a medical dressing. Neither adequately secures the tube. This drives even more unnecessary morbidity and mortality and carries multi-billion-dollar costs for hospitals and payers annually.
Solution ll
Robust medical capabilities in a single package solution
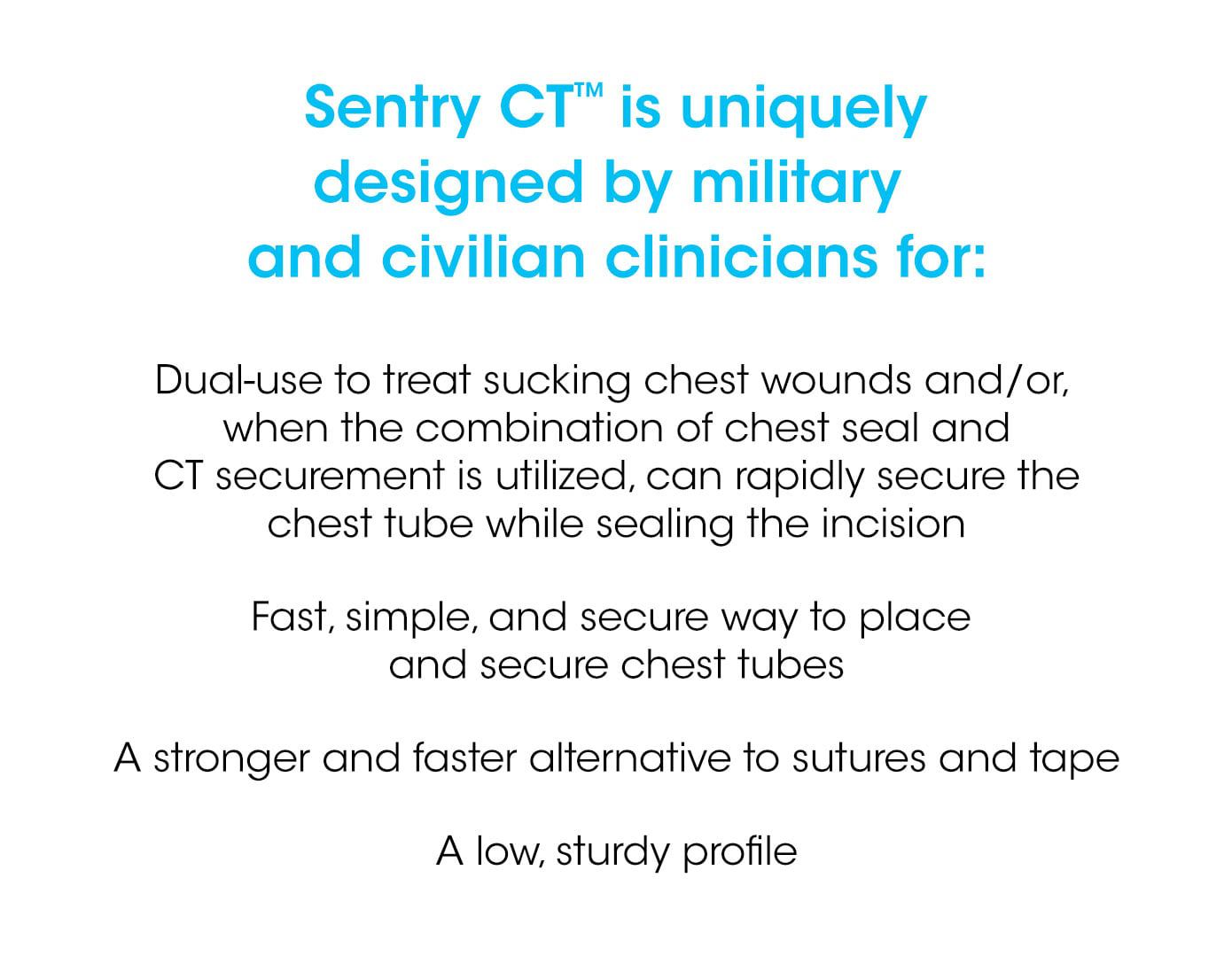
We've partnered with the US Military and the Mayo Clinic to create a novel dual-use device to seal chest incisions while providing a safe and efficient way to access a vented chest seal and secure a chest tube.

Product ll
Taking chest tube securement to the next level

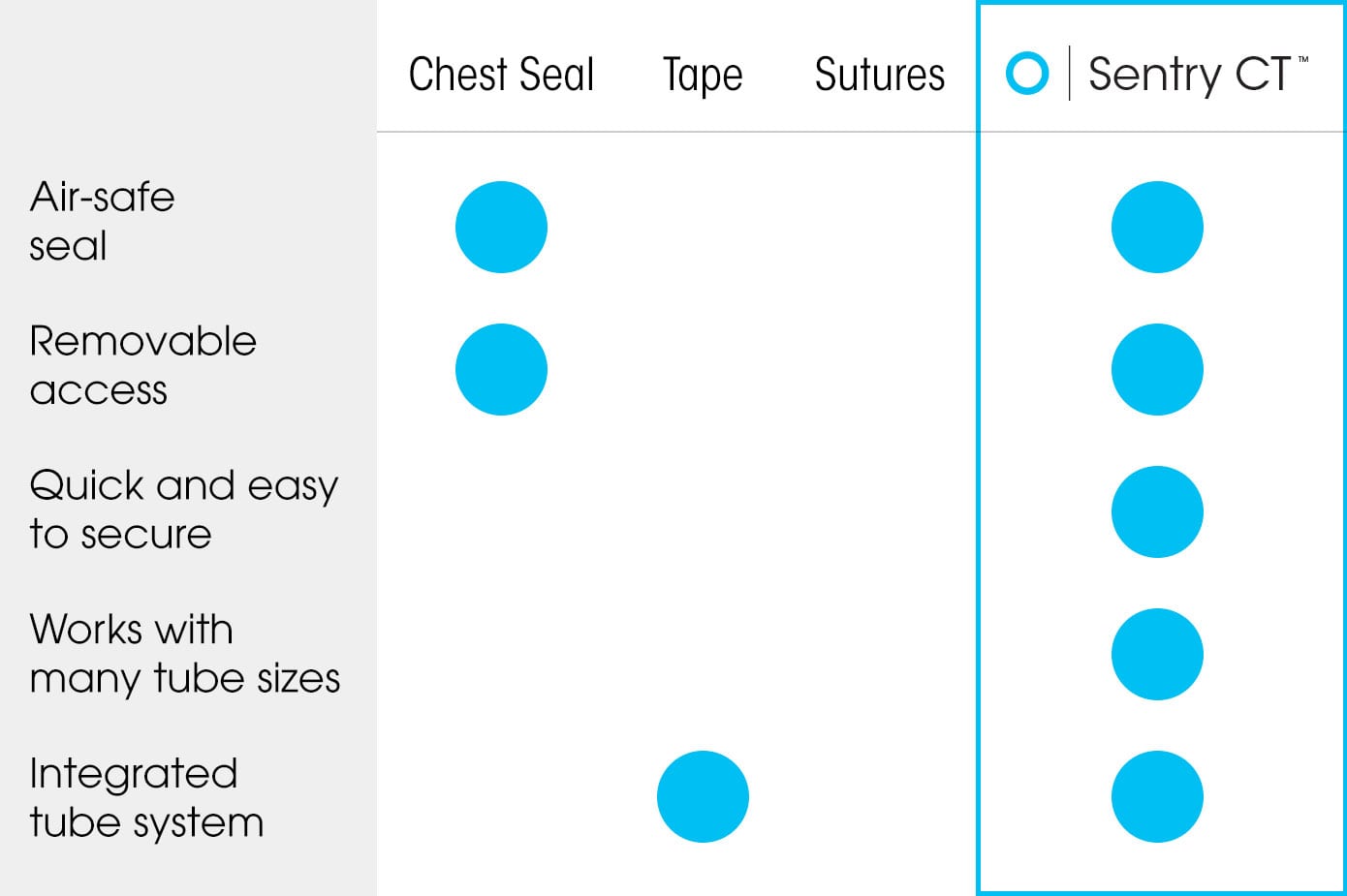
Our dual-use chest tube securement system allows for the prevention, management, and treatment of a collapsed lung that can be life-threatening. Sentry CT™’s vented chest seal acts as a one-way valve to allow air to exit the chest while preventing it from re-entering.
A novel silicone membrane maintains an airtight seal around a wide range of chest tube sizes. It also provides critical visual inspection ability and access to the incision site without compromising or contaminating the open wound.
The end-product is a quick and reliable chest tube securement that is 2X stronger than tape and sutures.
Traction
Primed for success
and ready to scale
Since our inception, we have captured the interest and support of leading clinician experts, engineers, researchers, healthcare institutions, and community partners.
Department of Defense Support for SolidAIRity Frontline™ and Sentry™ CT:
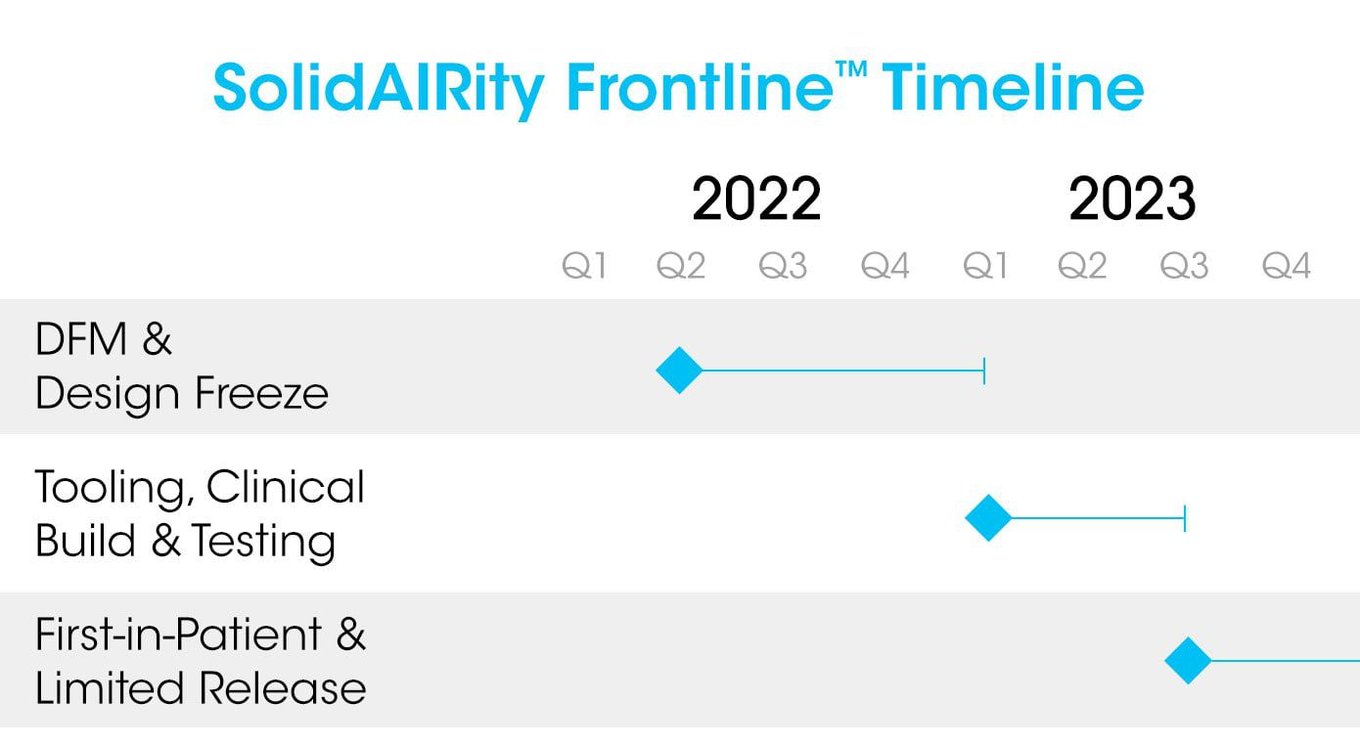
- We have successfully completed four Air Force SBIR contracts totaling $1.7M for the development of SolidAIRity Frontline™ and Sentry CT™, during which we received significant input from military providers during device design and development.
- Phase II awards and follow-on discussions with Department of Defense organizations signal continued interest in the development of manufactured products and future Phase III purchase contracts for both devices.

National Institutes of Health Support for SolidAIRity® Pediatric & SolidAIRity® Neonatal:
- We were recently awarded a $1.95M Fast-Track NIH Innovation Research grant to complete our novel pediatric and neonatal breathing tube securement products specifically designed to reduce unplanned extubation risk for the estimated 130K (US) mechanically ventilated children.
Multiple commitments for future
product evaluations and purchases
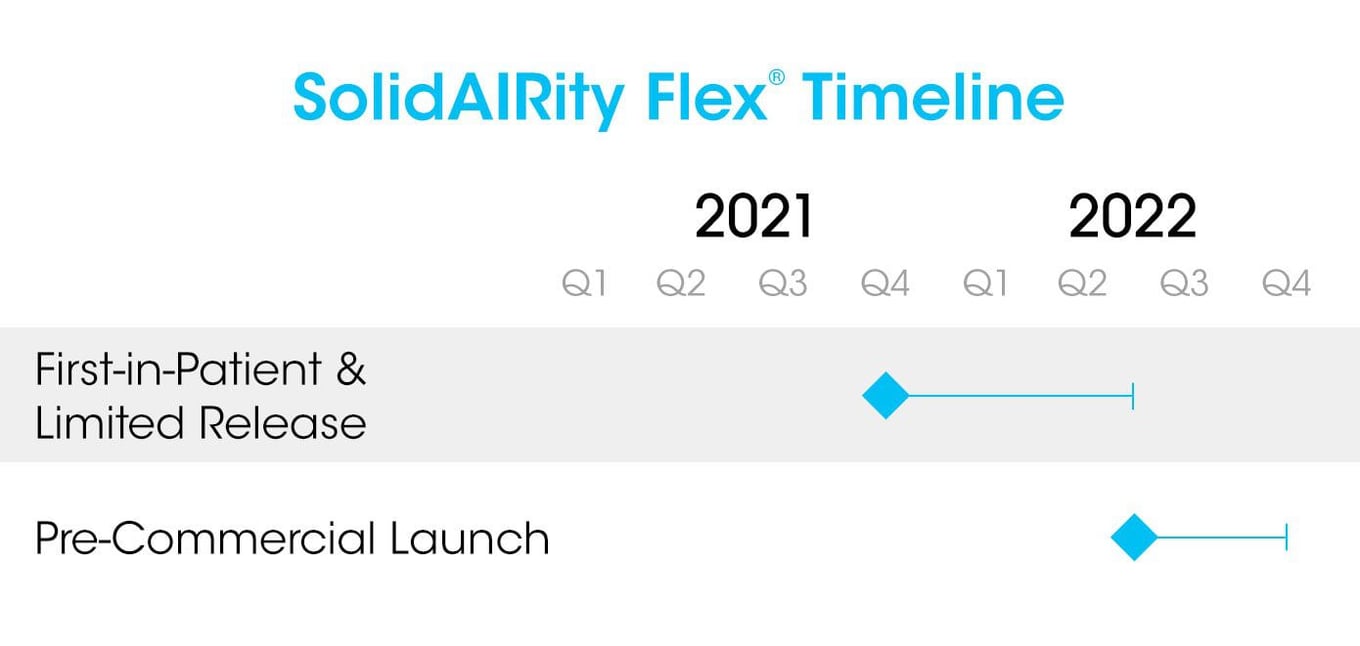
- Based upon preliminary clinical results from the first 74 patients at the University of Colorado Health Memorial, UCH Anschutz Medical Campus has agreed to a trial purchase of SolidAIRity Flex® devices for evaluation in October 2022.
- Common Spirit Health/ St. Joseph's Hospital in Arizona has agreed to a trial purchase of SolidAIRity Flex® devices for evaluation in Q4 2022 that is currently underway.
- The Veterans Health Administration has committed to evaluate adult SolidAIRity Flex® devices in a pilot project in Q4 2022.
- We are in ongoing discussions with U.S. Army Institute of Surgical Research regarding their interest to conduct a clinical study of our adult SolidAIRity Flex® device in the ICU and ED settings.
- And, we have executed a Letter of Intent with the country's largest air & ground ambulance provider, Global Medical Response, for clinical evaluations of SolidAIRity Frontline™ & SolidAIRity Flex®, with eligibility to be placed in the product catalog for purchase by clinical users nationally.
Hand-picked for world-renowned Mayo Clinic Arizona State University MedTech Accelerator
We were selected in a highly competitive process by the Mayo Clinic and Arizona State University to join their world-renowned MedTech Accelerator. We are now part of this exclusive community of medical device and healthcare IT companies with access to world-class physicians and experts to accelerate disruptive technologies and impact health outcomes that change people’s lives.

Award-winning company and
technology with global recognition
- 2022 Premier Breakthrough Technology Award Contract

- 2019 Colorado Bioscience Association Rising Star Company Winner for promising health innovations and business momentum.

- We were one of six (the only US company) World Airway Management (WAMM) Innovation Award Finalists, garnering strong international exposure for Securisyn® and SolidAIRity®.

- We were also awarded best poster presentation at the Special Operations Medicine Scientific Assembly for our research poster "Can Unplanned Extubation be Prevented? Introducing a Novel Airway Stabilization System for the Critically Ill SOF Patient".

Customers
We are creating raving fans
Securisyn® was founded to eliminate preventable harm and death from accidental tube dislodgements. Our customers are the caregivers and health facilities who treat patients with breathing and chest tubes.
Here's what they have to say about our products
"We have seen no instances of tube slippage requiring clinician depth re-adjustment upon placement of SolidAIRity Flex following post radiographic confirmation."
"At Mayo Clinic we want to translate idealism into action and work to invent the healthcare of tomorrow. This was the guiding vision for the formation of the MedTech Accelerator, a collaborative effort between Mayo Clinic and Arizona State University. We are so excited to have Securisyn Medical, a dynamic company with a truly innovative solution to the real and potentially tragic medical issue of Unplanned Extubation, as part of the program. We believe this program will accelerate their vision of bringing the number of this potentially tragic event to zero!"
"The S-Flex is easy to use, and the cheek pads hold better than the Hollister Anchorfast."
"SolidAIRity represents a completely unique and disruptive technology that will be unlike any other tube retention device on the market. When we saw this system for the first time, we both had a "Eureka!" moment where we were completely surprised by how elegantly the system worked and the completely new approach Securisyn has taken to reducing Unplanned Extubation."
"SolidAIRity is a much better solution than current devices."
We are obsessed with listening to our stakeholders and innovating on their behalf to develop products that keep patients safer, earn the trust of caregivers, and do right by our customers.
The simplicity and "magic" of our disruptive technology has attracted highly competent customers and partners:

Business model
Our go-to-market plan
We have a simple go-to-market plan for SolidAIRity Flex.
Our initial launch will be targeted to several large and influential hospital systems (University of Colorado Health, Common Spirit Health/St. Joseph's Hospital, Veterans Health Administration), and military research organizations (e.g. U.S. Army Institute for Surgical Research). We have been working closely with the key clinical leadership at these sites, and our existing commercial team will fully manage all product training and product conversion activities.
Following our initial limited market release, we will assess clinical and user feedback making recommended changes to the product and hospital onboarding process prior to full U.S. commercial launch. Securisyn Medical has signed a 5-year performance-based commercialization agreement with SunMed. Headquartered in Grand Rapids, MI, SunMed is a global manufacturer and distributor of consumable anesthesia and respiratory care products serving 97 countries worldwide. In partnership with SunMed Group Holdings, Securisyn will launch SolidAIRity Flex® to the U.S. Market in 2023 expanding to Rest of World markets in 2024.
As commercialization for SolidAIRity Flex® moves forward, Securisyn® will continue advancing our additional portfolio products.
Unplanned extubation is such a common and costly patient safety issue that many medical professional societies, patient safety organizations, and quality improvement organizations have joined in a collaborative to increase awareness and promote prevention activities. These include use of evidence-based best practice guidelines and bundles, research and publications, education, and quality improvement activities. We will also continue to strengthen and advance this clinical support network.

Key clinical & regulatory initiatives
- Conduct controlled clinical studies and/or objective trials of the products on a limited market release basis with established clinical partners
- Demonstrate that products are safe and effective for the intended use
- Validate usability of this next-generation device in a clinical setting under normal use conditions
- Establish improved patient outcomes through duration of care
- Demonstrate superior product performance over competitive benchmarks and current standards of care
Strategy and timeline
In 2019, Securisyn Medical® received FDA 510(k) clearance for its Class II flagship SolidAIRity® III Airway Stabilization System, which met all regulatory requirements for demonstrating safety and effectiveness in airway management of patients requiring oral intubation. Bench force studies comparing SolidAIRity® III to tape, twill, and leading commercial devices was completed as part of the FDA 510(k) submission to demonstrate superior restraint of ET tubes against 7x the amount of extubation forces. A limited market release of SolidAIRity® III further validated the intuitiveness and strength of Securisyn Medical’s Interlock™ restraint technology in a clinical environment with no unplanned extubations reported in patient cases across the continuum of care.
With applied learnings and support from key clinical partners and investors, Securisyn Medical® has transitioned away from the original integrated system (a proprietary breathing tube and corresponding stabilizer) and leveraged our core interlocking securement technology to innovate a line of breathing and other smooth tube securement devices with less complexity, faster regulatory path (Class I 510(k) exempt), and easier adoption for customers who are seeking a better securement solution to pair with their existing breathing tube products.
Securisyn Medical® products are driven by customer interviews and surveys to define both user needs and product requirements in early design. Despite being Class I, all products go through formal design controls and bench testing to evaluate system and subsystem performance under ranges of use conditions. Formative clinical user evaluations of the devices in simulated clinical environments are conducted with multiple areas of specialty (EMS, Emergency Medicine, Intensive Care, Respiratory Care, Anesthesia) to ensure critical needs and requirements are met.
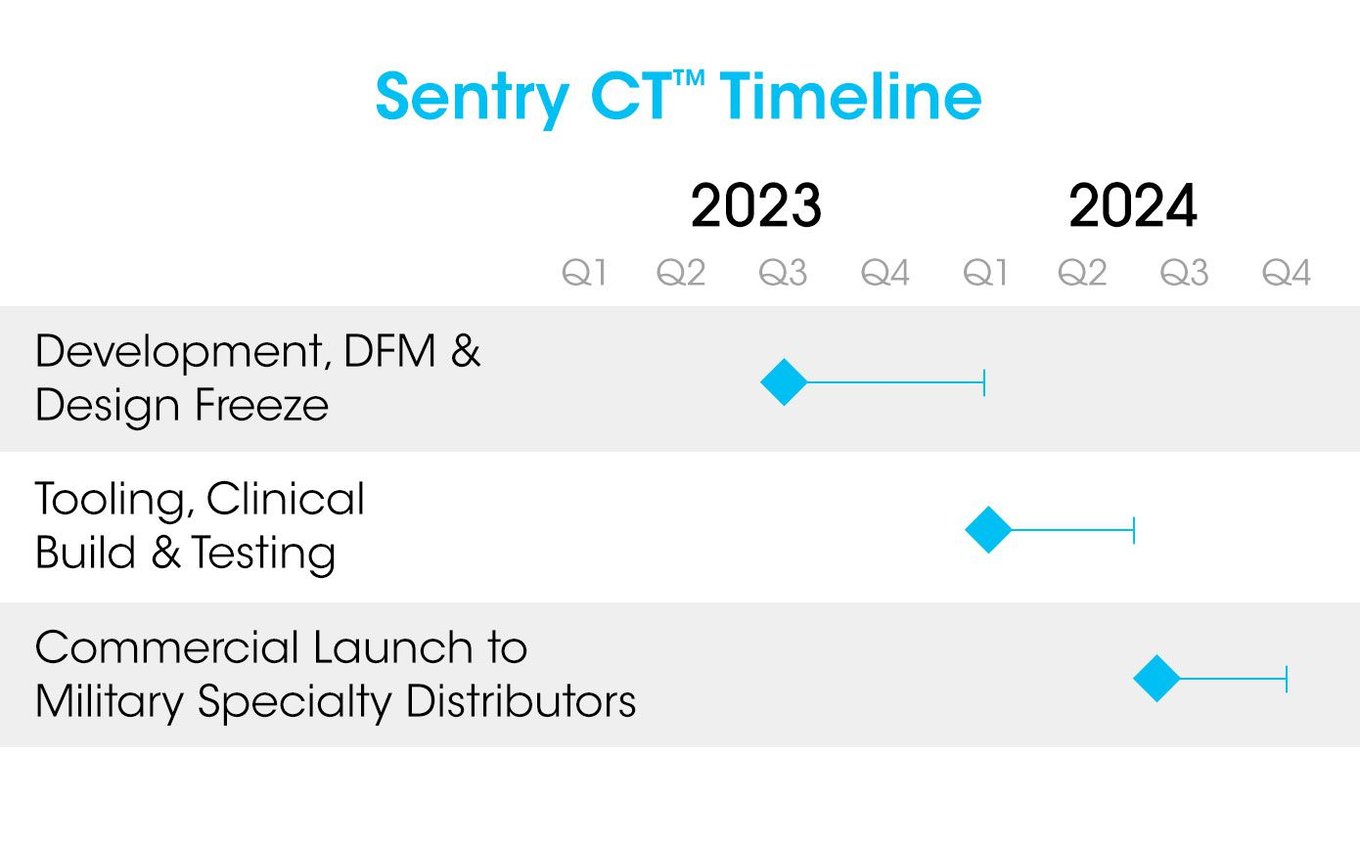
SolidAIRity Flex®, the company's latest oral endotracheal tube stabilization device, has initially launched in the United States, but the company is planning to file registration to distribute into Canada starting in 2023 and European Union by 2024. An extensive portfolio of both airway and medical tube securement products is at various stages of development with planned a cadence of commercialization spanning 2023-2025.
Quality & manufacturing operations
Early clinical build devices generated by low volume tooling and qualified manufacturing processes were employed to validate product design, usability, and customer preference in clinical phase. Securisyn Medical® utilizes approved medical-grade materials that meet the intended application and desired functionality to shorten product development cycles. Design for manufacturability (“DFM”) stages shall ensure minimal secondary processing and future manufacturing scalability. Low volume production tooling has been implemented support New Product Introduction (NPI) through clinical phase and into early commercial production in Year 1:
- Establish higher quality part production over soft tooling
- Validate mold layout and design before building production tooling
- Reduce design risk and upfront tooling expense while providing quicker time-to-market
- Provide a bridge between initial product builds and high-volume production
Scalability of new products is supported in development of a cost-effective manufacturing strategy to increase production capacity and reduce costs as volumes grow. High cavitation Class 101 molds shall maximize cost optimization efforts to achieve target costs of goods sold at higher scale volumes. Securisyn has secured a partnership with SunMed, a global manufacturer and distributor of anesthesia and respiratory care products, to scale low-cost manufacturing beginning in 2023.
Market
Attractive market opportunity with strong revenue potential and clear path to liquidity
In a growing $1.2B market, there are a lot of ways to transform how practitioners around the world give better care for their patients with smooth tube securement. Until now, our customers’ choice was “same old,” or better and more expensive. All that is about to change—because we have created a way forward that upgrades the industry to a new standard of care at a competitive price and a lower total cost of care.
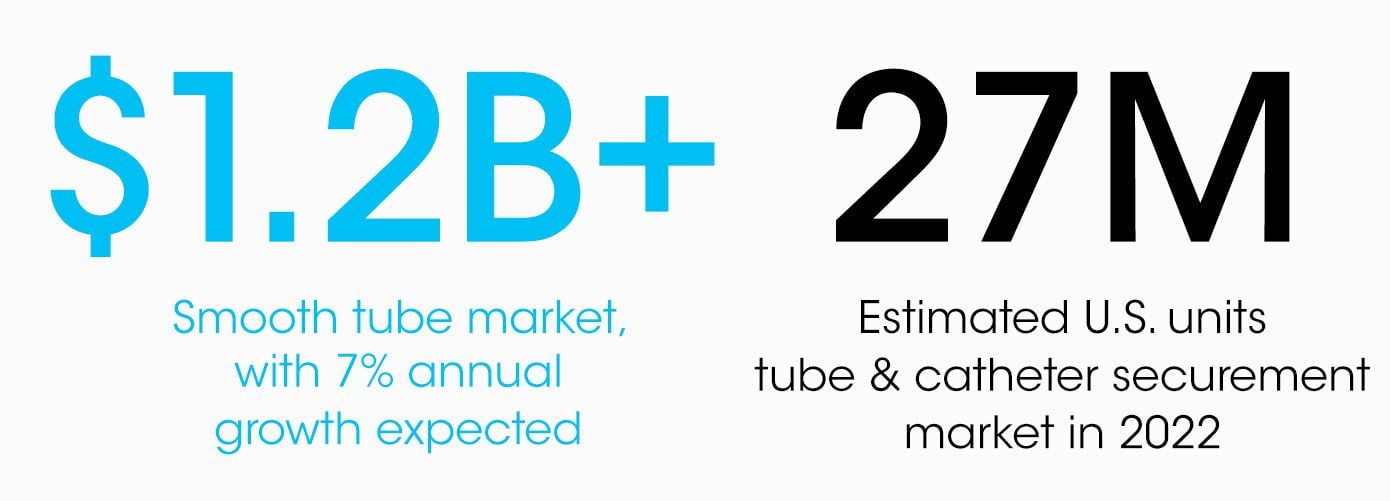
Our addressable tube & catheter securement market in the US is an estimated 27M units (8M outside of the operating room) in 2022, anticipated to grow 7% annually. Global unit potential is 2x that and exceeds 60M units annually.
Our tube securement portfolio has high strategic and commercial value, and offers an attractive revenue and growth opportunity of $7.7M, $14.3M, $21.7M, and $33.7M respectively in 2024, 2025, 2026 & 2027 on a stand-alone basis. This can be realized by driving market adoption with an established network of regional specialty sales & stocking distribution partners and internal clinical education sales support.
We plan to exit via M&A by a large-scale strategic selling synergistic products to same call points within 3-5 years. We have begun discussions with target acquirers and have received strong interest. Recent comps of healthcare industry product M&A transactions demonstrate ~7x TEV / Revenue multiples.
Competition
Competitive comparisons
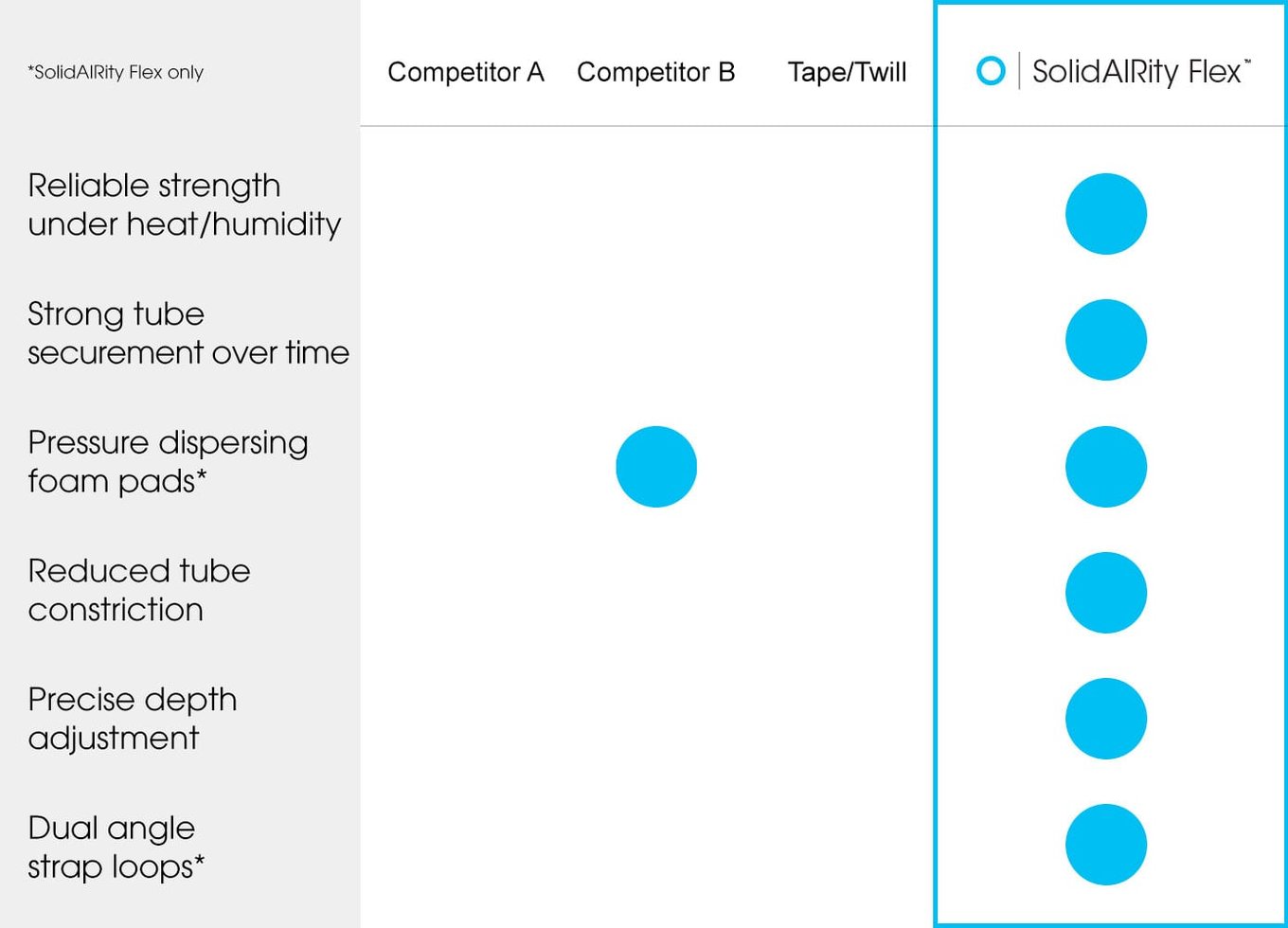
Tape and existing devices inadequately hold the tube against every day, real-world forces and continue to allow for unplanned extubation, where a breathing tube can be pulled out by a disoriented patient or dislodged during nursing care or diagnostic procedures. In contrast, SolidAIRity Flex® firmly secures the breathing tube from clinically significant movement and unplanned extubation.
SolidAIRity® vs other ET tube holders
Sentry CT™ vs other chest tube securement
Deep IP portfolio
Securisyn’s intellectual property portfolio includes 13 domestic and international patents, seven (7) issued, and numerous additional pending patent applications and IP in development. Its approved claims are broad, guarding its key differentiated technologies against infringement and competitive market entry.

Vision and strategy
A world free from the complications of inadequately secured tubes
During the time you've been reviewing this campaign, another life has been lost to unplanned extubation—perhaps even that of a young man like Drew who was taken from his family far too soon, or a US soldier in harm's way.
We believe one death is too many. That’s why Securisyn Medical® is partnering with The Do It For Drew Foundation, Airway Safety Movement, and the Patient Safety Movement Foundation to raise awareness and eliminate preventable deaths from accidental tube dislodgements.
With your support, we can make a difference.
Funding
$16M funded primarily by clinical professionals and healthcare systems
To date, we've raised significant funds from investors who are primarily accredited clinical professionals or healthcare systems that clearly understand the clinical need and the value of our disruptive solution.
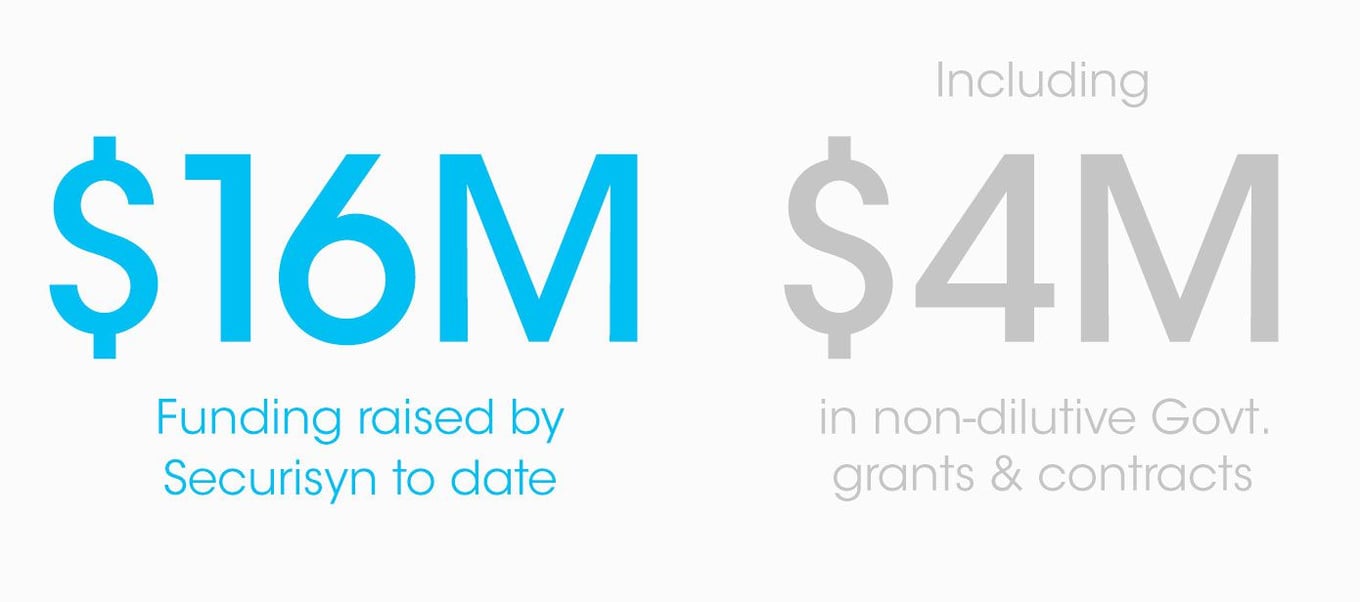
We are raising up to $5M to fund commercial launch and expanded adoption of our SolidAIRity Flex® device in 2023 and beyond.
Founders
Meet our team
Our team comprises seasoned clinicians and executives with a wealth of expertise in medical device development and commercialization from some of the largest and most reputable life science and healthcare companies: Abbott, Nellcor, Fisher & Paykel, Nuvasive, Tyco, Oridien, American Medical Response, and more.
Our actions and decisions always 'Start with Why' and our culture is deeply grounded in leadership principles we live by.

Leadership

Arthur Kanowitz, MD, FACEP
Co-founder, Chairman, Chief Medical Officer
Dr. Kanowitz is an Emergency Physician who has spent the last 16 years of his 47-year career in emergency medicine, working to improve patient safety during airway management. He is currently the chair of the Patient Safety Movement Foundation’s Airway Safety Workgroup, is the founder of the Airway Safety Movement and is the co-chair of the Society for Airway Management’s Coalition for Unplanned Extubation (UE) Awareness and Prevention. He is also on the Board of Advisors for the Patient Safety Movement Foundation.
Dr. Kanowitz retired from clinical practice in emergency medicine when he was appointed by Governor Hickenlooper to serve as the Emergency Medical and Trauma Services (“EMTS”) Medical Director for the State of Colorado from April 2008 to March 2017. He has served on numerous regional, state and national committees and councils and is diversely published in medical literature.

Mark Bruning
President & CEO
Mark is the company's President and Chief Executive Officer responsible for building the strategy and culture necessary to accomplish Securisyn Medical's singular mission to make airway management and other smooth tube & catheter securement safer and more effective through innovation, education, and collaboration. He is also responsible for building and leading the senior management team, for acquiring capital and market share, and for developing and realizing an appropriate growth strategy.
Under Mark's leadership, Securisyn has increased the diversity of its portfolio of intellectual property by inventing new products, securing key patents, and completing multiple iterative design improvements to existing technology that positions the company to significantly expand it's market size and opportunities. Securisyn's novel and patented adult endotracheal tube (ETT) stabilization device, SolidAIRity Flex, will launch with it's first early adopter hospitals in Q4 2021.
Mark has held numerous health care leadership roles, most recently leading a private equity owned pediatric home healthcare company to significant growth in 26 months, resulting in a successful exit. Previously, Mark served as President of American Medical Response (AMR), the nation's largest ambulance provider, as the first EMS caregiver to lead that organization. His primary interests focus on creating systems maximizing value creation in health care delivery, including leveraging existing and new technologies to take high value care to patients in more cost-effective and conducive settings.
Mr. Bruning holds a Masters of Business Administration degree from the Kellogg School of Management at Northwestern University. He also sits on several boards and community organizations, both non-profit as well as entrepreneurial business ventures in the telemedicine, NEMT digital marketplace solutions, homecare, and medical devices spaces.
Elyse Blazevich, MSOL
Co-founder, Manager
Elyse Blazevich is the President & CEO of the Colorado Bioscience Association. She co-founded Securisyn, and from its inception to 2021 was responsible for oversight of all daily operations of the business—including product development, manufacturing and supply chain, regulatory compliance, quality control, marketing, and general operations.
Backed by a Master’s degree in Organizational Leadership with a specialization in Applied Business Management, Elyse has successfully led the company from concept to commercialization and secured more than $15.0 million of private capital plus nearly $4.0 million of federal and state grant funding. Additionally, Elyse serves as an Editorial Advisory Board Member for Medical Product Outsourcing Magazine, as an alumna of the Colorado Bioscience Executive Leadership Program, and as Strategic Advisor and Past-Chair of the Young Professionals Board for Project C.U.R.E.
Bruno Darre
Independent Manager
Bruno Darré is the Founder of 73 Holdings, a private investment company primarily focused on control, growth equity and early-stage investments in operating businesses in the US with an emphasis on companies in the healthcare, consumer, general business, industrial and infrastructure industries.
Mr. Darré has over 20 years of private equity experience most recently as a Co-founder and Partner in Bow River Capital Partners, a Denver, Colorado based alternative asset management company with approximately $2 billion in assets under management making middle market buyout, real estate, energy and growth equity software investments.
Mr. Darré is the former Chairman of the Board of Professional Pediatric Home Care, Inc., and Lifecare Innovations, Inc., two healthcare portfolio companies majority owned and successfully exited by Bow River Capital Partners.

Troy Noem
Independent Manager
Troy Noem is a proven healthcare entrepreneur and executive with strong expertise in growing and scaling operations leading to successful M&A transactions at attractive multiples. Mr. Noem has been actively involved with Nuclear Care Partners since its founding in 2011, spending the first 4 years in a consulting relationship around business planning, expansion, and execution. He was brought on full-time in his current role of Chief Financial Officer in 2015, helping to drive strong growth and expansion across the US. Mr Noem is a 2001 graduate of South Dakota State University, with degrees in Mathematics and Physics.



 Oops! We couldn’t find any results...
Oops! We couldn’t find any results...